WORD
advertisement

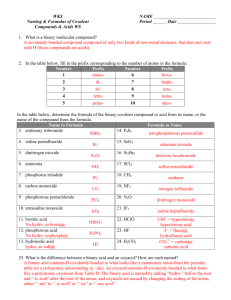
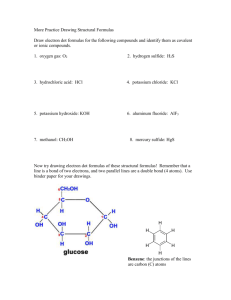
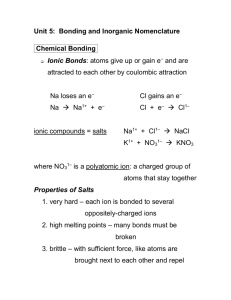
Ionic Binary The metal present can only form one kind of cation. Na only forms Na+ Cs only forms Cs+ Al only forms Al3+ Give the full name of the cation Give the root of the anion followed by the suffix –ide. For example: CaS Calcium sulfide KCl Potassium chloride MgO Magnesium oxide The metal present can form more than one kind of cation. It is necessary to say what kind of cation the metal has formed. Fe can form Fe2+ or Fe3+ Cu can form Cu+ or Cu2+ Pb can form Pb2+ or Pb4+ Give the full name of the cation Using a roman numeral give the oxidation state of the metal Give the root of the anion followed by the suffix –ide. For example: CuCl Copper (I) chloride Fe2O3 Iron (III) oxide PbCl4 Lead (IV) chloride Covalent Binary Compounds containing two nonmetals Give the full name of the first element Name the second element as if it were an anion in a Type One or Type Two compound. Use a prefix (i.e. mono, di, tri, tetra,…) to denote the number of atoms present. Note: The prefix mono is never used to in the name of the first element. CO is carbon monoxide, not moncarbon monoxide. For Example: N2O5 Dinitrogen pentoxide BF3 Boron trifluoride SF6 Sulfur hexafluoride Binary compounds are composed of just two elements. All binary compounds fall into three general categories, often called Type one, two, and three. It is not important to know that a compound is type one or two, but it is important to know the subtle differences between the two. The above are some rules for naming binary compounds based on the nature of the elements in it. Naming Polyatomic Ionic Compounds Polyatomic Ions Oxyanions Polyatomic Ions Names Ions can come in a variety of forms. Single charged elements like Li+ or Clare ions. Similarly several atoms can bond together and have a net negative or net positive charge. There are several series of polyatomic anions that contain a given element and a certain number of oxygen atoms. NH4+ Ammonium NO2- Nitrite NO3- Nitrate SO32- Sulfite SO42- Sulfate HSO4- Bisulfate (Hydrogen sulfate) OH- Hydroxide CN- Cyanide PO43- Phosphate HPO42- Hydrogen Phosphate H2PO4- Dihydrogen Phosphate CO32- Carbonate IO3- Iodate HCO3- Bicarbonate ClO- Hypochlorite ClO2- Chlorite ClO3- Chlorate ClO4- Perchlorate SCN- Thiocyanate C2H3O2- Acetate MnO4- Permanganate Cr2O72- Dichromate CrO42- Chromate O22- Peroxide There are several polyatomic anions and cations, and it is well worth it to commit them to memory. When there are two members in such a series, the name of the one with the smaller number of oxygen atoms will end with the suffix –ite. The anion with the larger number of oxygen atoms adopts the end suffix of –ate. In naming polyatomic compounds For example: Look at the compound, identify the polyatomic ion. Determine if it is an anion or a cation. Give the full name of the cation. If the cation is a metal, name it the same way it was done in Type One or Two binary compounds. Give the name of the polyatomic anion. CaSO3 Calcium sulfite CaSO4 Calcium sulfate When there are more than two members in such a series, the one with the least number of oxygen atoms get the prefix hypo- and the one with the greatest number of oxygen atoms gets the prefix per-. For example: For example: KNO3 Potassium nitrate Al(OH)3 Aluminum hydroxide PbSO4 Lead (II) Sulfate KClO Potassium Hypochlorite KClO2 Potassium chlorite KClO3 Potassium chlorate KClO4 Potassium perchlorate