Naming Chemical Compounds
advertisement

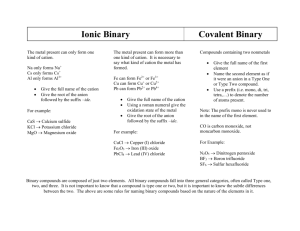
Naming Chemical Compounds • Binary Compounds of Metals with Fixed Charges (Ionic Compounds) • Covalent Compounds of Nonmetals: The Greek System • Binary Compounds of Cations with Variable Charges: Stock System Naming Chemical Compounds • Binary Compounds of Cations with Variable Charges: Common Name System • Polyatomics • Acids • Hydrates Binary Compounds of Metals with Fixed Charges • Name the cation first then the anion • Use the cation’s fixed charge directly from the periodic table (eg. Ca+2 is Calcium) • Name the anion using the root of the element’s name plus the suffix “ide” (eg. S-2 would be Sulfide) • Examples: • CaS would be Calcium Sulfide • Na2O would be Sodium Oxide • Potassium Chloride would be KCl Covalent Compounds of Nonmetals: The Greek System • Name the first element: if there are two or more, add a prefix to match the subscript • Name the second element also using a prefix to match the subscript (including “mono-” for one) • Prefixes: one=“mono-”, two=“di-”, three=“tri-”, four=“tetra-”, five=“penta”, six=“hexa-”, seven=“hepta-”, eight=“octa-”, nine=“nona-”, ten=“deca-” The Greek System • Example 1: N2O • Subscript for 2 is “di-” so it is dinitrogen • Subscript for O is 1 so the name is dinitrogen monoxide • Example 2: N2O5 • Subscript for nitrogen is 2 so it is dinitrogen • Subscript is 5 for oxygen or pentaoxide • The name is dinitrogen pentaoxide (pentoxide is also acceptable) Binary Compounds of Cations with Variable Charges: The Stock System • Named for German chemist Alfred Stock • Cations involved have at least two charges • Anion has one charge • Uses parentheses and roman numerals • Example: FeCl2 is iron(II) chloride The Stock System • Name the cation’s element first • Determine the charge by multiplying the anion’s charge by it’s subscript; then divide this by the cation’s subscript • Example 1: Name CuCl2 • Chloride anion’s charge is -1 times it’s subscript 2 equals 2 (Ignore sign of -2) • Divide by Copper’s subscript of 1 equals 2 • The result is Copper(II) chloride The Stock System • Example 2: Name Fe2O3 • Oxide anion’s charge is -2 times it’s subscript 3 equals -6 • Divide by Iron’s subscript of 2 equals 3 • The result is iron(III) oxide • Example 3: Give the formula for manganese(IV) oxide • The cation’s charge is given from the formula +4 • Oxide’s charge is -2; Since the charge of the final formula must be zero, two oxides or 2 times -2 equals -4 gives zero net charge • The result is MnO2 Binary Compounds of Cations with Variable Charges: The Common Name System • Proposed by Lavoisier “Father of Modern Chemistry” • Uses the Latin root of the cation plus –ous or –ic suffix to indicate oxidation state (-ous is lowest, -ic is highest) The Common Name System • Find the root name of the first element • Iron=“ferr-“, chromium="chrom-“, lead="plumb-”, tin="stann-“, copper="cupr-“, cobalt="cobalt-“, gold="aur-“, manganese="mangan-“, mercury="mercur-“ • Multiply the charge of the anion by its subscript (Ignore the sign) • Divide by the cation’s subscript • The lower of 2 values gives –ous; the higher -ic The Common Name System • Example 1: Fe2O3 • Multiply oxide’s -2 times the subscript 3 equals 6 (ignore the sign) • Divide 6 by iron’s subscript by 2 equals 3 • The result is ferric oxide (iron exists as +2 or +3) • Example 2: Cu2S • Multiply sulfide’s -2 times the subscript 1 equals 2 (ignore the sign) • Divide by copper’s subscript 2 equals 1 • The result is cuprous sulfide The Common Name System • Example 3: Ferrous Oxide • • • • Ferrous means +2 Oxide is -2 Use charge balance or criss-cross method to solve Fe+2O-2 yields FeO • Example 4: Stannic Phosphide • Stannic means Sn+4 • Phosphide is P-3 • Sn+4P-3 yields Sn3P4 Polyatomics • Naming can be either fixed or variable charges • When more than one polyatomic ion is required, parenthesis must be used • Example 1: Fe(NO3)2 • Decide if the cation has a variable charge (iron does so you must determine the roman numeral) • NO3 has a -1 charge so iron must be +2 • The name is iron(II) nitrate or ferrous nitrate Polyatomics • Example 2: Fe(OH)3 • Determine the charge of iron: Hydroxide is -1 so iron must be +3 • The compound is iron(III) hydroxide or ferric hydroxide • Example 3: Aluminum Phosphate • Aluminum is a fixed-charge of +3 • Phosphate ion is PO4-3 • The formula is AlPO4 Binary Acids • Binary acids are hydrogen with a non-metal (binary acids include: HCl, HF, HBr, HI) • “hydro-” STEM “-ic” is used (STEM comes from the anion) • The word acid is added at the end • Examples: • HCl is hydrochloric acid (STEM is chlor) • HF is hydrofluoric acid • HBr is hydrobromic acid Oxy (Ternary) Acids • Contains hydrogen and a polyatomic ion • Change the “ate” ending to “ic” and the “ite” ending to “ous” and add the word acid • Polyatomic ion that ends in “ate” • STEM –ic acid • Example HClO3 = chloric acid • Polyatomic ion that ends in “ite” • STEM –ous acid • Example HClO2 = chlorous acid Oxy (Ternary) Acids • Polyatomic ion with prefix “hypo” • Hypo- STEM –ous acid • Example HClO hypochlorous acid • Polyatomic ion with prefix “per” • Per- STEM –ic acid • Example HClO4 perchloric acid Hydrates • Compounds that have water in them • General Formula AB . xH2O • Name the compound (AB) and add the number of water molecules present (x equals the coefficient) • Examples: • CuSO4 . 5 H2O is copper(II) sulfate pentahydrate • MgSO4 . 9 H2O is magnesium sulfate nonahydrate Reference • See these web sites for practice!!! • http://dbhs.wvusd.k12.ca.us/webdocs/Nomencl ature/Nomenclature.html • http://www.woodrow.org/teachers/chemistry/lin ks/chem1/NamingComp.html