Ionic Compound Naming & Formula Writing Practice
advertisement

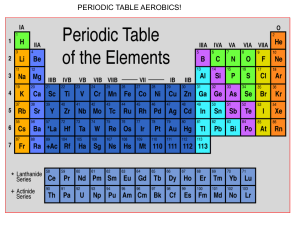
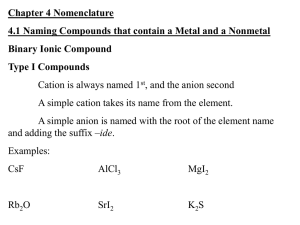
Naming and Writing Formulas for Ionic Compounds (Refer to slides—this is a shortened version of the notes) Background: Binary means two. Binary ionic compounds are composed of two elements. Binary Ionic compounds (simple) Cation name is followed by anion name. ex) potassium bromide (KBr) and lithium nitride (Li3N) Name a binary ionic compounds (with transition metals—THE DANGER ZONE!) Multiple charges on transition metals. Figure out the charge of the cation by first looking at the anion. It will only have ONE charge, so you can figure out the cation charge from that. We call this using a “position of strength” to determine the unknown charge. Ex) CuO. Copper can be +1 or +2, but oxygen is ALWAYS -2. Therefore, according to our formula, we have one copper atom that must balance out a -2 charge. That must mean that copper is +2 charge in this case. The name is copper (II) oxide or cupric oxide. Practice SnS2 _____________________________________ FeCl3 _____________________________________ SnF2 ______________________________________ CuI2 ______________________________________ CoI2 ______________________________________ CuI _______________________________________ Opposite process: Writing formulas for bionic compounds from their names. 1) write the symbol for the cation followed by the symbol for the anion. 2) Add whatever subscripts are needed to balance the charges Ex) Potassium chloride __________ Ex) Calcium bromide ____________ Using transition metals and other tricky situations: Ex) Iron (III) oxide. Iron is +3 and oxygen is -2. What do we do? My method that works for me (either LCD or criss-cross method) Solve for Iron (III) oxide using your preferred method: Ternary ionic compounds Ternary compounds contain atoms of three different elements. They usually contain a polyatomic ion. You need to memorize your polyatomics because you need to be able to recognize them quickly. Ex) calcium nitrate Procedure: 1. Write the symbol and charge for each. 2. Balance the charges by using subscripts. (Make sure to put the polyatomic between parentheses if you need more than one of them, because that shows that all of those atoms belong together and are treated as a unit. If you only need one of the polyatomics to balance everything out, then khalas, no parentheses.) Ex) Calcium carbonate Ex) Strontium sulfate Ex) Lithium carbonate Ex) potassium sulfate Ex) magnesium hydroxide Can you do it the other way? Writing formulas for ternary compounds from their names. Name the cation first, then the anion. Determine the charge of the polyatomic (it is your “position of strength”) and balance it out by adding an appropriate subscript for the cation. Ex) K2Cr2O7 Ex) LiCN Ex) Sr(H2PO4) Ex) (NH4)2C2O4 Ex) Fe(ClO3) Do some more practice!