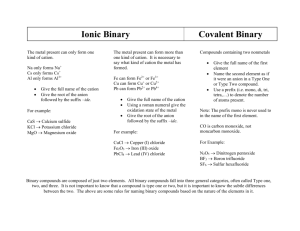
Chapter 5

Nomenclature
Chapter 5
1
Common Names - Exceptions
•
H
2
O = water, steam, ice
•
NH
3
= ammonia
•
CH
4
= methane
•
NaCl = table salt
•
C
12
H
22
O
11
= table sugar
2
Naming Starts with Classifying Compounds
•
Binary Compounds = only 2 elements
HCl NaBr Fe
2
O
3
CH
4
•
Compounds containing polyatomic ions
(NH
4
)
3
PO
4
Ca(MnO
4
)
2
•
Acids = formula often starts with H
HCl H
2
SO
4
HNO
3
3
Classifying Binary Compounds
•
Compounds containing a metal and a nonmetal are binary ionic
–
Type I = metal cation can only have one charge
–
Type II = metal cation can have different charges
•
Compounds containing two nonmetals
– Type III
• Compounds containing H and a nonmetal = Acids
4
Metal Cations
•
Type I
–
Metals that can only have one possible charge
– Determine charge by position on the Periodic
Table
–
AlCl
3
, BaO, Ca
3
N
2
•
Type II
–
Metals that can have more than one possible charge
– Determine metal cation’s charge from the charge on anion
–
FeCl
2
, FeCl
3
CuI, CuI
2
5
Table 5.1: Common Simple Cations and Anions
6
Type I Binary Ionic Compounds
•
Contain Metal Cation + Nonmetal Anion
•
Metal listed first in formula & name
•
Name metal cation first, name nonmetal anion second
•
Simple metal cation name is the metal name
– simple metals are Groups 1A, 2A and Al, Ag, Ga &
In
•
Nonmetal anion named by changing the ending on the nonmetal name to ide
7
•
Type I Binary Ionic Compound Examples
–
CaS
–
Calcium sulfide
–
AlCl
3
–
Aluminum chloride
–
Rb
2
O
– Rubidium oxide
– SrI
2
–
Strontium iodide
8
Type II Binary Ionic Compounds
•
Contain Metal Cation + Nonmetal Anion
•
Metal listed first in formula & name
• Name metal cation first, name nonmetal anion second
•
Metal cation name is the metal name followed by a
Roman Numeral in parentheses to indicate its charge
–
Determine charge from anion charge
–
Common Type II cations in Table 5.2
•
Nonmetal anion named by changing the ending on the nonmetal name to ide
9
Ion
Fe 3+
Fe 2+
Cu 2+
Cu +
Co 3+
Co 2+
Sn 4+
Sn 2+
Pb 4+
Pb 2+
Hg 2+
Hg
2
2+
Systematic Name Old Name
Iron(III)
Iron(II)
Copper(II)
Copper(I)
Cobalt(III)
Cobalt(II)
Tin(IV)
Tin(II)
Lead(IV)
Lead(II)
Mercury(II)
Mercury(I)
Ferric
Ferrous
Cupric
Cuprous
Cobaltic
Cobaltous
Stannic
Stannous
Plumbic
Plumbous
Mercuric
Mecurous
10
Determining the Charge on a Cation – Au
2
S
3
Determine the charge on the anion
Au
2
S
3 is -2
- the anion is S, since it is in Group 6A, its charge
Determine the total negative charge since there are 3 S in the formula, the total negative charge is -6
Determine the total positive charge since the total negative charge is -6, the total positive charge is +6
Divide by the number of cations since there are 2 Au in the formula & the total positive charge is +6, each Au has a +3 charge
Au
2
S
3
= gold(III) sulfide
11
•
Type II Binary Ionic Compound Example
–
CuCl
– Copper(I) chloride
– CoBr
2
–
Cobalt(II) bromide
–
PbO
2
– Lead(IV) oxide
– Hg
2
S
–
Mercury(I) sulfide
12
Figure 5.1: A flow chart for naming binary compounds.
13
Type III - Binary Compounds of 2 Nonmetals
•
Name first element in formula first, use the full name of the element
•
Name the second element in the formula as if it were an anion
–
However, remember these compounds do not contain ions !
•
Use a prefix in front of each name to indicate the number of atoms
•
Never use the prefix monoon the first element
14
Prefixes
Subscript
1
5
6
7
8
2
3
4
Prefix mono-
(not used on first nonmetal) ditritetrapentahexaheptaocta-
• Drop last “a” in the prefix if the name begins with vowel
15
•
Type III Binary Compounds
–
BF
3
– Boron trifluoride
– NO
–
Nitrogon monoxide
–
N
2
O
5
– Dinitrogen pentoxide
– CCl
4
–
Carbon tetrachloride
16
Compounds Containing
Polyatomic Ions
•
Polyatomic ions are charged entities that contain more than one atom
–
Must memorize name, formula and charge
– Look for Patterns!!
•
Polyatomic compounds contain one or more polyatomic ions
•
Name polyatomic compounds by naming cation and anion
– Non-polyatomic ions named like Type I and II
•
Polyatomic Acids contain H + and a polyatomic anion
17
Table 5.4: Names of Common Polyatomic Ions
18
•
Compounds with Polyatomic Ions
• Na
2
SO
4
•
Sodium sulfate
•
•
KMnO
4
Potassium permanganate
•
KH
2
PO
4
•
Potassium dihydrogen phosphate
•
•
(NH
4
)
2
Cr
2
O
7
Ammonium dichromate
• Fe(NO
3
)
3
Iron(III) nitrate
•
Cu(NO
2
)
2
•
Copper(II) nitrite
•
Mn(OH)
•
Manganese(II) hydroxide
•
•
CsClO
4
Cesium perchlorate
19
Patterns for Polyatomic Ions
Elements in the same column on the Periodic
Table form similar polyatomic ions
– same number of O’s and same charge
ClO
3
= chlorate
BrO
3
= bromate
If the polyatomic ion starts with H, add hydrogen - before the ions name and add 1 to the charge
CO
3
2= carbonate
HCO
3
= hydrogen carbonate
20
Patterns for Polyatomic Ions
•
-ate ion
– chlorate = ClO
3
-
• -ate ion plus 1 O
same charge, perprefix
– perchlorate = ClO
4
-
• -ate ion minus 1 O
same charge, ite suffix
– chlorite = ClO
2
-
• -ate ion minus 2 O
same charge, hypoprefix, ite suffix
– hypochlorite = ClO -
21
Acids
•
Contain H + cation and anion
•
Binary acids have H + cation and a nonmetal anion
•
Oxyacids have H + cation and a polyatomic anion
22
Table 5.5: Names of Acids that Do Not Contain
Oxygen
23
Acid
HNO
3
HNO
2
H
2
SO
4
H
2
SO
3
H
3
PO
4
HC
2
H
3
O
2
Name
Nitric acid
Nitrous acid
Sulfuric acid
Sulfurous acid
Phosphoric acid
Acetic Acid
24
Figure 5.3:
A flow chart for naming acids
25
Writing the Formulas from the Names
•
For Type III compounds, use the prefixes to determine the subscripts
•
For Type I, Type II, polyatomic
Compounds and Acids
– Determine the ions present
–
Determine the charges on the cation and anion
–
Balance the charges to get the subscripts
26
• Potassium hydroxide
•
KOH
•
Sodium carbonate
• Na
2
CO
3
• Nitric acid
•
HNO
3
•
Cobalt(II) nitrate
•
Co(NO
3
)
2
• Diphosphorous pentoxide
•
P
2
O
5
•
Ammonium sulfate
• (NH
4
)
2
SO
4
• Disulfur dichloride
•
S
2
Cl
2
•
Rubidium peroxide
•
Rb
2
O
2
27