Chemical Nomenclature
advertisement

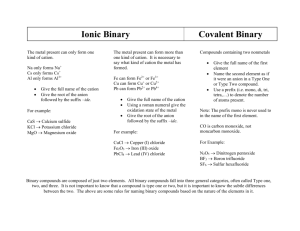
Ions and Ionic Compounds Remember an ion is an atom that has lost or gained electrons • Cations – positive – lost electrons • Anions – negative – gained electrons Ionic Compounds form when 2 or more atoms are joined by the loss and gain of electrons • ALWAYS cation + anion Cation is always first and anion is always second. Remember… • for the representative elements you can use the location on the periodic table to determine how many electrons will be lost or gained. Ex. Hydrogen is +1 Ex. Fluorine is -1 For the transition elements you need more information. Transition Elements show their charge by the roman numeral that follows their name • Ex. Copper (I) is Cu+1 Transition elements always lose electrons so their charge is ALWAYS positive. The name of cations (positive ions) is the SAME as the name of the element. Ex. K+1 is potassium • Transition metals do need the charge as a Roman numeral following the name. So, if the element is in the middle – it needs a roman numeral! Ex. Cu+1 is copper (I) The name of anions (negative ions) is different. Anions end in –ide. • Ex. F-1 is fluoride (fluorine) Copper(II) Potassium Barium + Sulfur = + Nitrogen = + Sulfur = Lithium + Oxygen = Calcium + Nitrogen = Copper(II) + Iodine = Potassium + Sulfate = Magnesium + Hydroxide = Ammonium + Sulfite = Calcium + Phosphate = Aluminum + Nitrate = Potassium + Chromate = Rubidium + Perchlorate = Potassium + Permanganate = Lead(IV) + Hydroxide = Compound must be neutral • Charge must equal ZERO. The Sum of the cation and anion charges must be zero. • Ex. (+1) + (-1) = 0 • Ex. (+2) + (-2) = 0 We needed one cation and one anion to make the sum ZERO. If the charges do not add up to be zero, then you must add additional cations or anions so that the sum does equal zero. • Ex. (+1) + (-2) ≠ 0 so… you must add another (+1) ion. • (+1) + (+1) + (-2) = 0 We needed TWO (+1) cations and ONE (-2) anion to make the sum ZERO. The number of cations and anions you need to make the sum ZERO is the ratio of cations to anions in an ionic compound. This ratio is called the FORMULA UNIT for the ionic compound. The ratio is represented in a formula as subscripts for the cation and anion. Since the charges add up to equal zero, NO CHARGE should be written in the formula. Copper(II) + Sulfur = • Cu+2 + S-2 = CuS Potassium + Nitrogen = • K+1 + N-3 = K3N Barium + Sulfur = • Ba+2 + S-2 = BaS Lithium + Oxygen = Calcium + Nitrogen = Copper(II) + Iodine = Potassium + Sulfate = Magnesium + Hydroxide = Ammonium + Sulfite = Calcium + Phosphate = Aluminum + Nitrate = Potassium + Chromate = Rubidium + Perchlorate = Potassium + Permanganate = Lead(IV) + Hydroxide = Name cation first • Remember Transition Metals should have a roman numeral representing the charge in the name. Name the anion second. • Elements that are anions will always end –ide. • Do not change the ending of a polyatomic ion. Ex. Fluorine Fluoride (ending change) Ex. Sulfate Sulfate (no change) Cation + Anion Ions are joined by the transfer of electrons • Creates Electrostatic forces (attraction of opposite charges) that hold the ions together Ionic Compounds are composed of a continuous arrangement of oppositely charged ions. • NOT a single separate unit Bonds between atoms will be ionic when there is a LARGE difference in electronegativity between the atoms. • > 1.7 ∆EN Metal + Nonmetal • Opposite sides of the periodic table – large differences in electronegativity Metal + Nonmetal (usually) Crystalline SOLIDS at room temp. (most) • Crystal – repeating geometric pattern Brittle HIGH melting points HIGH boiling points • Some are so high it takes extreme conditions to get them to change to gas Conduct electrical currents when melted or dissolved in water Molecules and Covalent Bond Compounds that form when atoms SHARE electrons • Forms a covalent bond NEVER contain ions • NEVER have charges Contain only nonmetals Nonmetal + Nonmetal Can be solids, liquids, or temperature gases at room • State is determined by the bond strength (compounds with stronger bonds tend to be solids) LOW melting points LOW boiling points POOR conductors of electricity under any conditions Can be Polar or Nonpolar • Depends on how the electrons are shared • Polar compounds are better conductors Since molecules do not have charges, their names and formulas are determined differently. Two elements may form covalent bonds in more than one way creating DIFFERENT chemical compounds • Ex. H2O and H2O2 Other examples: • CH4 and C2H6 • C6H12O6 and C12H22O11 Whenever two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. • Ex. H2O and H2O2 contain the same mass of Hydrogen The mass of Oxygen is in a ratio of 1:2 Since two elements can combine in more than one way, we must use information besides their identities to determine their formulas and names. • Scientists perform quantitative analysis to determine the mass ratios of elements to determine their formulas • We can use electron dot diagrams (Lewis structures) to help us determine possible arrangements Names • Elements are named in the order they appear in the formula. • The final element’s ending is changed to –ide. • Prefixes are added to each element to identify the number of each atom in the formula EXCEPTION: Mono- is not used for the first element. If there is only 1 of the first element, no prefix is added. Prefixes 1. Mono2. Di3. Tri4. Tetra5. Penta- 6. Hexa7. Hepta8. Octa9. Nona10.Deca- The prefixes in the name can be used to write the formula for a given compound. • Symbols for each element are written in the order they appear. • Subscripts are added based on the prefix of each element. CO2 • Carbon dioxide CCl4 • Carbon tetrachloride N2O5 • Dinitrogen pentaoxide Carbon Monoxide • CO Sulfur dioxide • SO2 Diphosphorus • P2S3 trisulfide Acids are molecular compounds that behave more like ionic compounds ALWAYS contain Hydrogen • Hydrogen is always the first element in the formula Can contain polyatomic ions Since they form compounds like ionics but consist of only nonmetals, there are special rules for naming and writing formulas for acids. Formulas for acids are determined the same as ionic compounds. • Sum of the charges must equal ZERO • Hydrogen is always +1 • Element or polyatomic ion it combines with must be NEGATIVE Example: Hydrogen • H+1 + Cl-1 = HCl Example: Hydrogen • H+1 + SO4-2 = H2SO4 + Chlorine + Sulfate Two different ways to name acids • Hydrogen + Element Ex. HCl • Hydrogen + Polyatomic Ion H2SO4 Name begins with Hydro• Ex. Hydro Root of the element • Ex. Hydrochlor Ends with –ic acid • Ex. Hydrochloric acid Practice • HF • H2S Name begins with polyatomic ion Root • Sulf- Name ends with –ic acid or –ous acid • -ic acid is used when the polyatomic ion name ends with –ide or –ate Sulfuric acid (H2SO4) • -ous acid is used when the polyatomic ion name ends with –ite Sulfurous acid (H2SO3) Practice • • • • HClO3 HCN HClO2 H3PO3 Metallic Bonds Metal + Metal Consists of positive metal ions with a “sea of electrons” • Electrons are free floating – are not attached to any one atom or ion HIGH melting points HIGH boiling points HIGH conductivity Malleable, Ductile, High luster (shine) • All properties are a result of the “free” electrons. 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. sodium hydroxide sodium bromide barium hydroxide calcium oxide lithium sulfide carbon monoxide sulfur dioxide iron (II) sulfate silver (I) chloride copper (II) hydroxide ammonium sulfide nickel (II) fluoride mercury (I) sulfate iron (III) oxide 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. magnesium phosphate nickel (II) carbonate diphosphorous pentoxide aluminum phosphate nitrogen dioxide phosphorus trichloride dinitrogen pentoxide germanium tetrachloride scandium bromide bromine monoiodide antimony pentasulfide