Chemexam Review--organic naming and properties

Organic Naming and Properties
1.
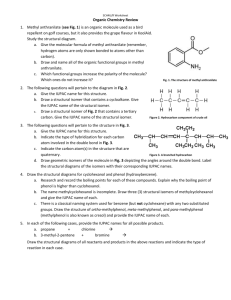
Which is the correct IUPAC name for the following molecule? a) cis-N-chloro-N-ethyl-5-amino-4-ethyl-2-pentene b) N-chloro-N-ethyl-1-amino-2-propenylbutane c) cis-N-chloro-N-ethyl-1-amino-2-ethyl-3-pentene d) cis-N-chloro-N-ethylamino-2-heptene
2.
Match the following compounds to their names
A B a) A. Butanal & B. isopropanol b) A. Butanone & B. isopropanol c) A. Butanone & B. 2-propanal d) A. Butanal & B. propanone
3.
The correct prefix for a 1, 2 numbering on a benzene ring is: a) m b) o c) p d) s
4.
Which diagram represents N-bromo-N-cyclopentyl-2-cyclopentylethanamide? a) b) c) d)
5.
An alkene has at least one of which type of bond? a) C-C b) C=C c) C=O d) C-N
6.
Which of the following is 1,2-difluoro-4-hexanol? a) b) c) d)
7.
If the following molecule is an ester, what intermolecular forces would be present? i. London Dispersion ii. Dipole-Dipole iii. Hydrogen Bonding a) i & iii b) i, ii & iii c) ii & iii d) i & ii
8.
Name the following compound. a) t-butyl-propylperoxide b) t-butyl-propyl-peroxide c) propyl-t-butyl peroxide d) propyl-t-butylperoxide
9.
The following molecule is: a) trans-3-pentene b) pentene c) cis-2-pentene d) cis-3-pentene
10.
Which molecule is correctly matched with its IUPAC name and common name? a) b) c)
11.
How many double bonds does a benzene ring have? a) 1 b) 2 c) 3 d) 0 e) 6
d)
12.
_F_ T/F Ethers have London dispersion, dipole-dipole and hydrogen bonds and have lower melting points then hydrocarbons.
13.
_T_ T/F Carbonyls have decreased molecular forces and they are polar.
14.
Another name for an aromatic is a(n): a) Ether b) Alcohol c) Ester d) Benzene
15.
What is the name of this molecule? a) 5-chloro-4-cyclobutylhexanoic acid b) 1-chloro-2-cyclobutylhexanoic acid c) 2-chloro-3-cyclobutylhexan-6-ol-6-one d) 1-hydroxyl-4-butyl-5-chlorohexanaldehyde