3 Organic Chemistry Review Fall 2012
advertisement
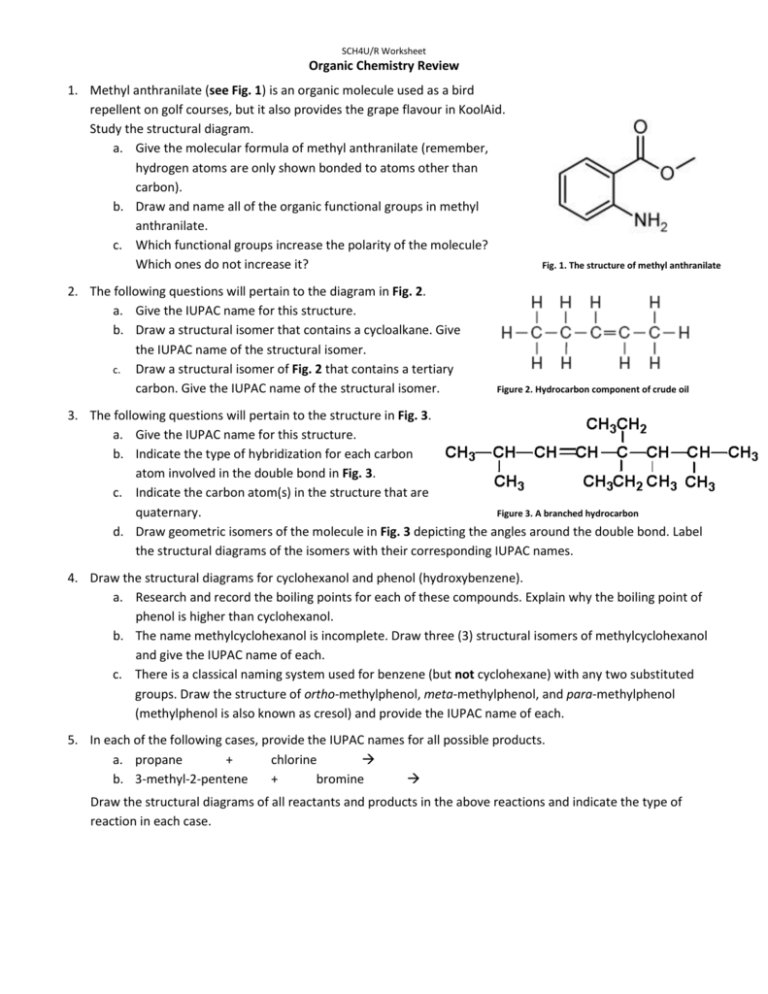
SCH4U/R Worksheet Organic Chemistry Review 1. Methyl anthranilate (see Fig. 1) is an organic molecule used as a bird repellent on golf courses, but it also provides the grape flavour in KoolAid. Study the structural diagram. a. Give the molecular formula of methyl anthranilate (remember, hydrogen atoms are only shown bonded to atoms other than carbon). b. Draw and name all of the organic functional groups in methyl anthranilate. c. Which functional groups increase the polarity of the molecule? Which ones do not increase it? 2. The following questions will pertain to the diagram in Fig. 2. a. Give the IUPAC name for this structure. b. Draw a structural isomer that contains a cycloalkane. Give the IUPAC name of the structural isomer. c. Draw a structural isomer of Fig. 2 that contains a tertiary carbon. Give the IUPAC name of the structural isomer. Fig. 1. The structure of methyl anthranilate Figure 2. Hydrocarbon component of crude oil 3. The following questions will pertain to the structure in Fig. 3. a. Give the IUPAC name for this structure. b. Indicate the type of hybridization for each carbon atom involved in the double bond in Fig. 3. c. Indicate the carbon atom(s) in the structure that are quaternary. Figure 3. A branched hydrocarbon d. Draw geometric isomers of the molecule in Fig. 3 depicting the angles around the double bond. Label the structural diagrams of the isomers with their corresponding IUPAC names. 4. Draw the structural diagrams for cyclohexanol and phenol (hydroxybenzene). a. Research and record the boiling points for each of these compounds. Explain why the boiling point of phenol is higher than cyclohexanol. b. The name methylcyclohexanol is incomplete. Draw three (3) structural isomers of methylcyclohexanol and give the IUPAC name of each. c. There is a classical naming system used for benzene (but not cyclohexane) with any two substituted groups. Draw the structure of ortho-methylphenol, meta-methylphenol, and para-methylphenol (methylphenol is also known as cresol) and provide the IUPAC name of each. 5. In each of the following cases, provide the IUPAC names for all possible products. a. propane + chlorine b. 3-methyl-2-pentene + bromine Draw the structural diagrams of all reactants and products in the above reactions and indicate the type of reaction in each case. 6. Alcohols that are heated in the presence of an acid catalyst can be converted into alkenes with the release of water. Complete the following reaction by including the structural diagram(s) of all possible product(s) and by providing the IUPAC names of reactants and products. a. an alkene + water b. Indicate the type of reaction this represents. c. Explain why the alkene has a much lower boiling point compared to the reactant. d. The conditions for this reaction are similar to a condensation reaction. Draw the structural diagram and provide the name of the organic family to which the condensation product belongs. 7. Draw the structural diagram and provide the IUPAC name of the compound being described in each case. a. the product of the controlled oxidation of 1-pentanol b. the alcohol that must be oxidized to form 4-methyl-2-heptanone c. the product of the controlled oxidation of 3-methyl-2-butanol d. the product of the reduction (hydrogenation) of cyclohexanone 8. Many organic molecules are used to produce artificial flavours (and aromas). The molecule shown in Fig. 1 exhibits a fruity flavour and is often used to produce a pineapple flavour. a. Write the IUPAC name of the molecule shown in Fig. 1. b. To what family of organic molecules does it belong? Describe the functional group that characterizes this organic family. c. Provide the IUPAC name of the carboxylic acid and the alcohol that could be used to form the molecule in Fig. 1 through a condensation reaction. Figure 1. Molecule that produces pineapple flavour d. Using structural diagrams for reactants and products, show the chemical equation of the condensation reaction that would produce the molecule in Fig. 1. Indicate the reaction conditions that are required.