Submitted by: Md.Saiful Islam
advertisement

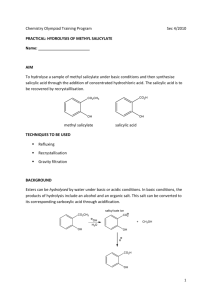
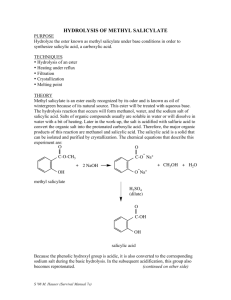
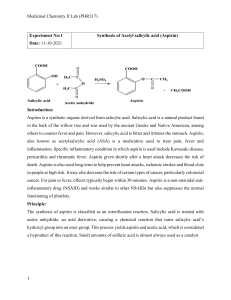
Page 1 of 5 A Practical Note Book on Medicinal Chemistry-III Lab Course code: BPM00414 Submitted to: Mr. Muhammad Rakib Al-Mamun Senior Lecturer Department of Pharmacy Manarat International university Submitted by: Md.Saiful Islam ID: 0915BPM00485 Department of pharmacy Manarat International University Submission date: 23.02.2013 signature: Page 2 of 5 Index Experiment No 01 Name of the Experiment Synthesis of Methyl Salicylate (Oil of wintergreen) Page No 3-5 Date Page 3 of 5 Experiment No: 01 Name of the Experiment: Synthesis of Methyl Salicylate (Oil of wintergreen). Principle: The reaction of carboxylic acid and alcohol produces an ester and water. The reaction is catalyzed with acid. Easter often have a fruity taste or odor. Here methyl Salicylate, prepared using methanol as the alcohol, will give smell like wintergreen. Reaction: + CH3OH Salicylic Acid Methanol + H2 O Methyl Salicylate Reagents: i. ii. iii. iv. v. vi. Dry salicylic acid. Pure methanol. Concentrated sulfuric acid. Distilled water. 5% sodium bicarbonate. Anhydrous calcium chloride. Apparatus: i. ii. iii. iv. v. vi. vii. viii. Round bottom flask. Reflux condenser. Separating funnel. Stand. Ice bath. Measuring cylinder. Electric balance. Distillation apparatus. Procedure: i. ii. iii. iv. The reflux apparatus is set using a 250ml round bottom flask. 0.05mole i.e. 6.9gm of salicylic acid is taken in the round bottom flask and 30ml of methanol is added. 8ml of concentrated H2SO4 is added into it gently. The flask is swirled gently to thoroughly mix the reactants. Page 4 of 5 v. vi. vii. viii. ix. x. xi. xii. xiii. xiv. A reflux condenser is attached to the round bottomed flask and using a heating mantel, the mixture is refluxed gently for two hours. The reaction flask is cooled by immersing it in ice water bath. The content of the flask is poured into 100ml of cold distilled water contained in a 400ml beaker. The reaction mixture is placed in a separating funnel, to determine the aqueous layer 50% sodium bicarbonate solution is added. The mixture is placed washed gently swirling. The aqueous layer is discarded. The organic layer is poured into an Erlenmeyer flask and dried with anhydrous calcium chloride. The ester is decanted and the mass of the product is recorded. The ester is combined with other groups for distillation into a distillation flask by filtering through a funnel with a small cotton plug. Methyl Salicylate boils at 2220c. This is very high temperature to do a distillation; in order to do this a low pressure distillation is set up by attaching a water aspirator to the place on the apparatus. The fraction between 110-1150c is collected in a separate flask. Pure oil of wintergreen is obtained. Calculation: Molecular weight of salicylic acid = 138gm Molecular weight of methyl Salicylate = 152 gm. 138gm of salicylic acids yields =152 gm of methyl Salicylate 6.9gm of salicylic acids yields = 152 × 6.9 gm of methyl Salicylate 138 = 7.6 gm of methyl Salicylate. From the experiment, we get 6.81 gm of methyl Salicylate from 6.9 gm salicylic acid. Percentage of yield product = = Practical Value × 100 Theoritica l Value 6.81 × 100 7 .6 = 89.61% Page 5 of 5 Result: Percentage of yielded methyl Salicylate is 89.61. Precautions: i. ii. iii. iv. v. All of the apparatus should be handled carefully. All of the chemicals should be handled carefully. All of the measurement should be done carefully. Product should not be adhered at the side of the flask. Boiling stones should be used.