Redox Review Game: Chemistry Practice
advertisement

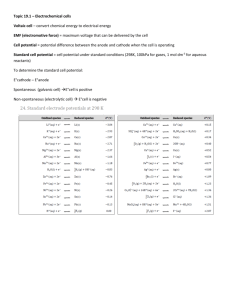
Redox Review (You will need to use page 712 in your textbook for this review game) Multiple Choice: 1. What is the oxidation number of Cr in Cr2O3? a. +6 d. +2 b. +3 e. –2 c. –3 2. What is the oxidation number of Cr in H2Cr2O7? a. +6 d. +2 b. +3 e. –2 c. –3 3. What is the oxidation number of Cr in CrSO4? a. +6 d. +2 b. +3 e. –6 c. –3 4. Investigate the following: 2Sr + O2 2SrO Complete the following sentence: ______ is reduced and ______ is oxidized. a. Sr ; O b. O ; Sr c. O2 ; SrO d. SrO2 ; Sr e. Sr O2 5. In a reaction between sodium metal and zinc chloride, ______ is reduced and ______ is oxidized. (Hint: sodium has a lower electronegativity than zinc). a. Na ; Zn b. Zn 2+ ; Na c. Na ; Na d. ZnCl2 ; Na e. none of these are correct because this reaction does not occur. 6. Calculate E0cell to determine whether the following redox reaction is spontaneous as written: Zn (s) + F2 (g) 2F1- (aq) + Zn2+ (aq) a. No the reaction is not spontaneous because the cell potential is 3.6278V b. No the reaction is not spontaneous because the cell potential is 1.5325V c. Yes the reaction is spontaneous with a cell potential of 3.6278V d. Yes the reaction is spontaneous with a cell potential of –5.6335V e. No the reaction is not spontaneous with a cell potential of –3.2531V 7. Which reaction would create a better battery? reaction 1: 2Li (s) + Co2+ (aq) 2Li+ (aq) + Co (s) reaction 2: 2I1- (aq) + K+ (aq) I2 (g) + K (s) a. reaction 1 b. reaction 2 c. neither reaction would create a battery d. both are equally good batteries 8. The following equations are used to make an electrolytic cell. Which reaction occurs at the anode? i. Br2 (l) + 2e- 2Br1- (aq) ii. Na+ (aq) + e- Na (s) a. equation i b. equation ii c. neither – these reactions cannot create an electrolytic cell. 9. The following equations are used in a galvanic cell. Which reaction occurs at the anode? i. Cd2+ (aq) + 2e- Cd (s) ii. MnO41- (aq) +8H+ (aq) + 5e- Mn2+ (aq) + 4H2O (l) a. equation i b. equation ii c. neither – these reactions cannot create a galvanic cell. 10. In fuel cells, ____ act as fuel (Mark all that apply) a. Oxygen c. zinc b. Hydrogen d. carbon dioxide 11. Which of the following statements is not true of Redox reactions? a. Oxidation must be accompanied by a reduction reaction b. Redox involves a loss and gain of electrons c. Involves a change in oxidation states of the elements involved d. Redox occurs in all reactions. 12. True or False? A lithium battery is used in cell phones and electric cars since lithium has the highest standard electrode potential of the metallic elements. a. true b. false 13. The oxidation number of N in NH3 is –3. This means (Mark all that apply) a. that the nitrogen has a negative three charge b. the hydrogen does not have any electrons around it c. the nitrogen is partially negative but does not have a charge. d. this is an ionic compound e. –3 is a number we assign N so we can track electrons. 15. What is the purpose of a salt bridge? (Mark all that apply) a) Allows the circuit to be complete in order to allow electrons to travel. b) Allows the oxidized metal to move to the other side so the ions can become a solid. c) Allows ions to move so the salt in the salt bridge can be oxidized. d) Allows ions to move to neutralize the beakers so they do not get a build up of charges. 14. What are the products for the following unbalanced reaction? (Mark all that apply.) NiCl2 + Na a) NaCl2 b) Ni+2 c) Ni d) NaCl e) This reaction does not occur 16. Which of the following are characteristics of both electrolytic and voltaic cells? (Mark all that apply) a) an anode and cathode are present. b) a battery is required to make the cell work properly. c) A salt bridge must be present to make the cell work. d) A wire must connect the anode to the cathode in order for the cell to work. 17. A cell is constructed by immersing a strip of lead in a 1.0M Pb(NO3)2 solution and a strip of silver in a 1.0M AgNO3 solution. The circuit is completed by a wire and a salt bridge. As the cell operates, the strip of silver gains mass (only silver), the strip of lead loses mass, and the concentration of lead ions increases in the solution around the lead strip. Which of the following represents the reaction that occurs at the cathode in this cell? a) Pb 2+ Pb + 2eb) Pb Pb2+ + 2ec) Ag Ag + + ed) Ag + + e- Ag 18. Under standard conditions, what is the standard cell potential for the cell? Cd|Cd2+ || Cu2+|Cu? a. 0.74 V b. -0.74 V c. -0.06V d. 0.06V 19. How many moles of electrons have been transferred between the species in the following reaction? 2Cr3+(aq) + 3Cu(s) → 2Cr(s) + 3Cu2+(aq) a) 2 c) 4 b) 3 d) 6 20. a) b) c) Why do fuel cells not run down as batteries do? Fuel is provided from an outside source Fuel cells do not use chemical reactions Fuel cells do not require an electrolyte 21. a) b) c) d) An example of electrolysis is (Mark all that Apply) The flow of current from a primary battery The charging of a secondary battery Corrosion of an iron pipe Addition of hydrogen gas to a fuel cell. 22. The electrolysis of an aqueous sodium chloride solution using inert electrodes produces gaseous chlorine at one electrode. At the other electrode, gaseous hydrogen is produced, and the solution around the electrode becomes basic. Which of the following equations is the correct equation for the cathode half-reaction in this electrolytic cell? a) 2Cl- Cl2 + eb) 2H2O + 2e- H2 + 2OHc) Cl2 + 2e- 2Cld) H2 + 2OH 2H2O + 2 e23. Electron flow is from the cathode to the anode in an electrolytic cell. 1. True 2. False 24. Nonmetals are usually good reducing agents for metals a. True b. False Balancing Redox Reactions 1. Balance the following in basic solution: NH3 (g) + O2 (g) NO (g) + H2O (l) 2. Balance the following in acidic solution: H6TeO6 (aq) + Br2 (g) TeO2 (s) + BrO31- (aq) Voltaic and Electrolytic Cells 1. Draw the voltaic cell composed of a Zn/Zn2+ half-cell and a Ni/Ni2+ half-cell. Label the Zn, the Ni, the anode and the cathode. Draw an arrow in the direction of the electron flow. Show the flow of positive and negative ions in the salt bridge. Label the electrodes as positive or negative. Show the ions in solution. c. Write the short hand notation for the reaction that occurs. d. What is the maximum potential that can be achieved, using 1.0 molar solutions at 298K and 101 kPa? 2. In the following set-up, an aqueous solution of NiSO4 is electrolyzed. At the cathode, a whitish-gray solid substance appears, and the pH is constant. At the anode, the pH drops, and bubbles of gas form. Label the drawing with the anode, cathode, ion flow, electron flow and any gases and show equations for both the anode and cathode half-reactions.