Emission Spectroscopy - Furness
advertisement

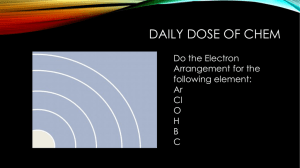
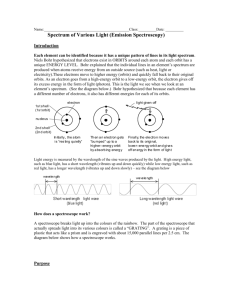
Name Lab: Emission Spectroscopy Purpose Demonstrate an understanding of the relationship between atomic structure and emission spectroscopy Observe continuous and bright-line spectra Identify elements by comparing your results to a published reference Materials: Spectroscope colored pencils various light sources computer Introduction: In their natural state, the electrons near the nucleus have less energy than electrons in higher energy levels. Adding energy in the form of heat, light, or electricity can energize some of the electrons and make them move to a higher energy level. The electrons cannot stay at the high energy state (excited state) for long, and they drop back down to their regular level (ground state). But remember, energy cannot be created or destroyed; it can only be transferred from one object to another or changed from one form to another. When the electron drops from its excited state back to the ground state, the extra energy is given off in the form of a photon. A photon is a set amount of electromagnetic energy in the form of light. The energy of this photon depends on the difference in energy between the electron’s ground state and excited state. Each electron emits its own characteristic energy. The energy that is lost by each electron is given by Max Planck’s equation, E = hv, where E is energy, h is a constant, and v is the frequency of the photon. Energy is added (heat, light, electricity) Ground State Excited State Energy is given off in the form of photons Ground State A spectroscope is a device that splits light up into its different frequencies. Each frequency is visible as a unique color. If the light emitted by energized elements is passed through a spectroscope, a pattern of narrow lines of light is produced. Because every element has its own pattern of electrons in energy levels, each element produces its own distinct pattern that differs from the pattern of every other element. The particular pattern of frequencies of light emitted by an atom is referred to as its emission spectrum, or bright-line spectrum. The emission spectrum can be used to identify an element, just as fingerprints and DNA are used to identify people. In this experiment, you will use a spectroscope to examine spectra from different light sources. By comparing your results to a known reference, you can determine what gases were in the light bulbs. Pre-Lab Questions (Complete sentences) 1. What is the normal low energy state of electrons called? 2. How do electrons become energized? 3. What is this high energy condition called? 4. What happens to them after they get to the higher energy state? 5. What happens to their extra energy? How to use a spectroscope: Hold the scope flat with the label on top. Look through the round hole. There is a slit on the far right on the scope. Aim that slit at the light source until it is bright. When it is aimed correctly, look to the left of the slit at the measurement scale (ruler). Depending on the light source, you should see either a continuous spectrum (rainbow) or some individual colored lines. On your paper, draw what you see. Make sure you put the correct number of lines in the correct place. Procedure: 1. Using a spectroscope, examine the spectrum emitted by an incandescent light bulb (round bulb). Using colored pencils and lightly shading, reproduce what you see on your data sheets. Make sure the colors are in the right location on the wavelength scale. 2. Repeat the procedure for a fluorescent light (tube ceiling light). Look carefully for anything new in the spectrum and make sure to show it in your data sheet. 3. Use the spectroscope to examine the spectra emitted by the special bulbs in the center of the classroom. Draw what you see. Analysis questions (Complete sentences) 1. Compare and contrast the spectra from the incandescent and fluorescent light. Be specific. 2. Compare and contrast spectra from incandescent light and the special bulbs. 3. What causes the different colors of light in the spectra? 4. Get your laptop and google “emission spectra of elements”. Click on the “chemlinks/beloit” site. Your bulbs were hydrogen, helium, neon and mercury, but not in that order. Use the spectra to determine which bulb was which and write your answer in your lab book.