Hydrogen Spectra & Flame Tests Lab Worksheet
advertisement

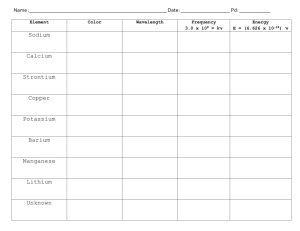
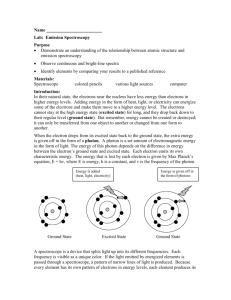
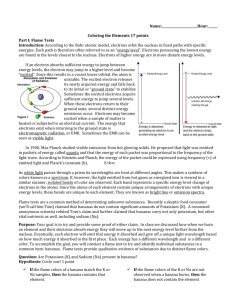
Date Period Name Hydrogen Spectra Using a spectroscope, observe the line spectrum of hydrogen and draw it to scale (add numbers), in color, in the space provided. 6 points Wavelength (nm) Observe at least two other spectra and list the colors observed. 4 points substance Bulb color Colors observed with spectroscope Metal ions give off characteristic colors when they are put in a flame. Carefully light the Bunsen burner. Use a wet wooden splint to collect some salt containing the metal ion to be tested. Record the first bright color observed. 14 points Metal ion Barium Calcium Copper Lithium Potassium Sodium Strontium Descriptive Flame Test Color Elemental Electron Configuration Now test your assigned unknowns. They each contain one metal ion you tested above. 4 points Unknown Color Post – Lab Questions 1 point each 1. Which pair of ions were the hardest to tell apart? Identity 2. What color(s) would you expect to see for a sample of the following compounds: potassium chloride, potassium nitrate, potassium sulfate? 3. If milk was boiling on a gas stove and it boiled over, what color would you expect to see? ______4. The characteristic bright-line spectrum (color) of an element is produced when A. electrons are emitted by the nucleus as beta particles B. electrons move to higher energy levels C. electrons are gained by an atom D. electrons fall back to lower energy levels Define the following terms: 5. Ground state 6. Excited state 7. Photon 8. Quantum Read pages 792-795 in your text book (Modern Chemistry). 9. Compare your results to the pictures of the flame tests. Be sure to talk about each metal. 10. What elements from the lab could be used for the fireworks shown? 11. The emission spectrum of strontium is given in Angstroms. 1 Angstrom is 1 x 1010 m. Calculate the frequency of the red line. 12. What element not tested in this laboratory is used in flares?