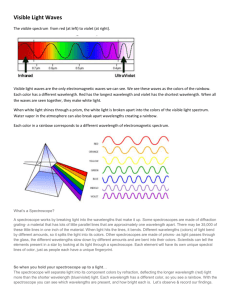
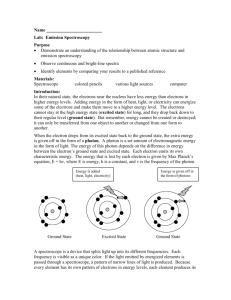
Atomic Hydrogen Spectrum Lab Report
advertisement

Title: Atomic Hydrogen Spectrum Lab Date: 8/31/09 Purpose: To observe the emission spectra of hydrogen and two other gases. Materials: Spectroscope, power supply, gas tubes. Research Question: Do all the gas tubes have the same spectrum? Hypothesis: Procedure: Calibration of spectroscope 1. Place the slit opening of your spectroscope close to the Helium discharge lamp, as directed by your instructor. 2. Adjust the slit of the spectroscope so that seven lines are visible. 3. Record the scale readings for all the visible lines on the data sheet. 4. Make a scatter plot of wavelength versus scale reading. Emission spectrum of Hydrogen 1. Move your spectroscope to a hydrogen discharge lamp. 2. Adjust the slit of the spectroscope so that three intense lines are visible. 3. Record the scale readings for all the visible lines on the data sheet. Note: a faint line at 410 nm may not be visible. Data: Calibration of wavelength scale with Helium emission lines Line Color Wavelength Scale (nm) Reading 1 Red 707 2 Red 668 3 Yellow 558 4 Light 501 green 5 Darker 492 green 6 Blue471 green 7 Blue447 violet DRAW A SCATTER PLOT OF HELIUM DATA WITH A REGRESSION LINE. Hydrogen Emission Lines Line Color Scale Wavelength(nm) Reading 1 2 3 4 410 USE THE SCATTER PLOT TO DETERMINE Wavelength(nm) DATA. ______________________ Emission Lines Line Color Scale Wavelength(nm) Reading 1 2 3 4 Conclusions: