Honors Chemistry Name ______________ Spectroscopy: Element
advertisement

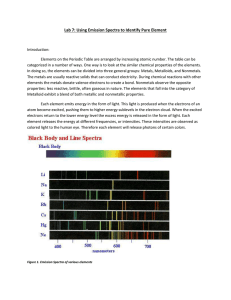
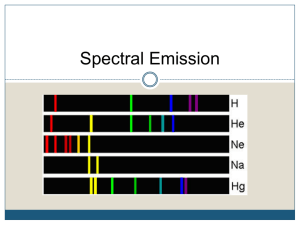
Honors Chemistry Name ________________ Spectroscopy: Element Identification and Emission Spectra http://www.800mainstreet.com/spect/emission-flame-exp.html#top Read the introductory material and study the diagrams before performing the procedure for Part 1. You may want to copy the diagrams/equations found in this section into your notebook. Part 1: Flame Tests & Identification of an Unknown Metal Metal ion Observed Flame color barium _________________________ calcium _________________________ sodium _________________________ rubidium _________________________ potassium _________________________ lithium _________________________ Unknowns Flame color Identity of metal ion based on flame test Unknown 1 ____________ __________ Unknown 2 ____________ __________ Part 2: Observing Line Spectra with the Spectroscope View the emission line spectra for the elements in this section, and answers the questions below. How do these emission spectra compare in terms of colors and numbers of emission line positions? Are the spectra identical? What if anything is similar? What is different? Examine the spectra for the elements Na, Ne, Hg, or He and answer the following questions. 1) What is the element with the greatest number of visible emission lines: What element has the longest wavelength in the spectrum of this atom in nanometers: What is the color of light for this longest wavelength: 2) What is the element with the fewest number of emission lines: What is the longest wavelength in the spectrum of this atom in nanometers: What is the color of light for this longest wavelength: Post-Lab Questions: 1) What “new” idea did you learn from this experiment? 2) Why does a sodium vapor street light look yellow instead of white? 3) What would you expect to happen to the size (volume) of a hydrogen atom when the outer electron moves from n=2 shell to the shell with n=4? Would the volume increase? View Extra Line Absorptions & Emissions @ http://jersey.uoregon.edu/vlab/elements/Elements.html