Lab 7: Using Emission Spectra to Identify Pure Element
advertisement

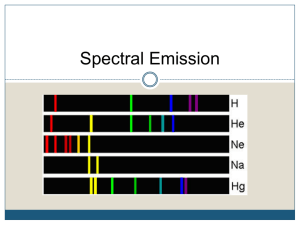
Lab 7: Using Emission Spectra to Identify Pure Element Introduction: Elements on the Periodic Table are arranged by increasing atomic number. The table can be categorized in a number of ways. One way is to look at the similar chemical properties of the elements. In doing so, the elements can be divided into three general groups: Metals, Metalloids, and Nonmetals. The metals are usually reactive solids that can conduct electricity. During chemical reactions with other elements the metals donate valence electrons to create a bond. Nonmetals observe the opposite properties: less reactive, brittle, often gaseous in nature. The elements that fall into the category of Metalloid exhibit a blend of both metallic and nonmetallic properties. Each element emits energy in the form of light. This light is produced when the electrons of an atom become excited, pushing them to higher energy sublevels in the electron cloud. When the excited electrons return to the lower energy level the excess energy is released in the form of light. Each element releases the energy at different frequencies, or intensities. These intensities are observed as colored light to the human eye. Therefore each element will release photons of certain colors. Figure 1. Emission Spectra of various elements Using this knowledge emission spectra can be developed for each element to indicate what wavelengths of light are released by each element. Every element’s emission spectrum is different, so it can be used to identify various elements. The bands represent the wavelengths of light emitted by the element when excited electrons drop to lower energy levels. Materials: Bunsen Burner pipette Spatula Discharge Tube Watch glass Discharge Tube power source Inoculation Loop Spectroscope Alcohol (preferably methanol) Colored pencils/markers/crayons Various salt compounds Methods: A. Flame Test 1. Light the Bunsen burner at the station. Make sure the flame is at a safe distance from all and that the bench is clear of any other materials. 2. Using the plastic pipette add 3-4 drops of alcohol to the watch glass. 3. With the spatula, add a small scoop of the salt solution to the alcohol on the watch glass. DO NOT LET THE SPATULA TOUCH THE ALCOHOL, this will cause contamination. 4. Using the Inoculation Loop mix the alcohol and salt. 5. Dip the Inoculation Loop in the solution and suspend it over the inner cone of the Bunsen Burner’s flame. 6. Record any and all color changes in the flame observed. Be specific: “emerald green”, not just “green”. Or “red with spots of orange”, not just “reddish”. 7. Repeat procedure 3 times for each solution to make certain of your data. Do this at all stations. B. Emission Spectra 1. With the lights off observe and record the color of each discharge tube, making note of the element and record your data. Be descriptive as possible. 2. Using the spectroscope observe the emission spectrum of each gas and record the emission on diagrams below. Pay special attention to the wavelengths and colors of each emission line. Be as exact as possible. 400nm 500nm 600nm 700nm 800nm Figure 2. Diagram of emission spectra C. Identifying Unknown 1. Repeat the process for the unknown gases in the discharge tube. Use your other emission spectra to compare the unknown to. 2. If you cannot determine the unknown gas look up the spectra for various gases and identify it. Support your answer with your data and research.