Emission Spectra Lab
advertisement

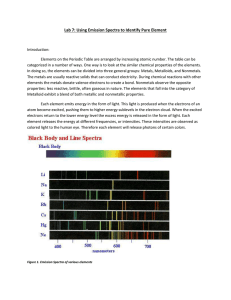
Today I will be able to: Explore how the photoelectric effect creates atoms with unique atomic spectra Identify the anatomy of electromagnetic waves Informal assessment – monitoring student interactions and questions as they complete the lab Formal assessment – analyzing student responses to the lab and exit ticket Common Core Connection Build Strong Content Knowledge Reason abstractly and quantitatively Use appropriate tools strategically Evaluate: Warm-Up Explain: Emission Spectra Notes Engage/Explore: Emission Spectra Lab Elaborate: Star Spectra WS Evaluate: Exit Ticket Write the abbreviated electron configurations for the following: Au Br- What is wrong with this abbreviated configuration for Cl? [He]2s22p63s23p5 Today I will be able to: Explore how the photoelectric effect creates atoms with unique atomic spectra Identify the anatomy of electromagnetic waves Finish Energy, Wavelength, Frequency Calculations Wear closed toe shoes Friday (B-day), Monday (A-Day) Warm – Up Emission Specta Notes Emission Spectra Lab Wavelength, Energy Frequency Calculations Exit Ticket Electrons can be move as waves When electrons release energy, release in the form of light photons Study of the emission and absorption of light by atoms and molecules Wavelength (λ - lambda) – distance between crests in a wave Frequency (v - nu) – number of complete waves passing a point in a given amount of time Amplitude - distance from wave origin to peak or crest Electrons are found on specific energy levels When electrons absorb energy, they more to a higher energy level When electrons emit energy, they move to a lower energy level and release light energy Photoelectric Effect Electrons are emitted from solids, liquids or gases when they absorb energy from light. Color is seen if the electron falls back to energy level (n) 2 This is visible light The amount an e- fall determines the frequency of EM radiation released (n = 1 = UV; n = 3 infrared) No two elements give off the same spectra of colors, because they vary in the number of protons and electrons Emit their own unique line spectra Can be used to identify elements Emission Spectra of Hydrogen 1 nm = 1 x 10-9 m = “a billionth of a meter” Continuous All wavelengths of visible light are seen Line (Emission) Spectra Only specific bands of color are seen 1 nm = 1 x 10-9 m Rotate from Station to Station to observe line spectrum of different elements and complete the lab. Complete the worksheet when you finish your lab. c = λν ◦ C= 3.0 x 108 m/s (speed of light) ◦ λ = wavelength (meters) ◦ ν = frequency (Hertz or 1/seconds) Planck came up with the equation E= hν E = Energy (Joules) h = Planck’s Constant 6.626 x 10-34 J*s ν = frequency (Hertz or 1/seconds) Complete the practice at your desk. Ask Ms. Ose for help if you have questions. What is happening to the electrons when we see color on the emission spectrum? Revisit the Blue Electron Exam Study Guide and Complete the boxes covered in class today