Activity 1: Spectra from various sources
advertisement

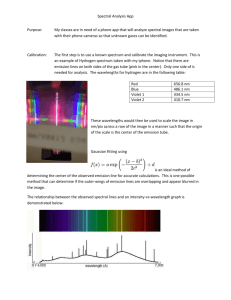
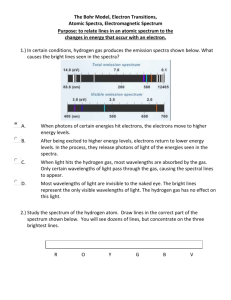
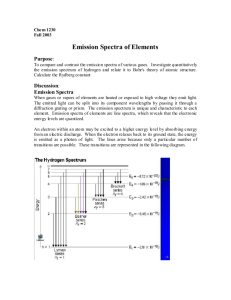
Activity 1: Spectra from various sources Look through the spectroscopes at each of the light sources. Off to the right you should see a spectrum superimposed on a scale. Using the colored pencils, record the spectra you see, using the handout that will be available in lab. (The units are 10-5 cm or 10-7 m). CAUTION: The emission tubes are powered by a 5000V source, which could provide a nasty shock to anyone touching the metal supports! DO NOT TOUCH! Looking at an incandescent light bulb: 4 5 6 1. What range of wavelengths corresponds to: Blue? Yellow? Green? 2. 7 8 Red? Orange? Is there a distinct division between the various colors, or do they simply fade into the next color? Looking at a Hydrogen (H) emission tube 4 5 6 7 8 6 7 8 6 7 8 6 7 8 Looking at a Helium (He) emission tube 4 5 Looking at a Neon (Ne) emission tube 4 5 Looking at a Mercury (Hg) emission tube 4 5 3. Is there a distinct division between the various colors, or do they simply fade into the next color? 4. Are the observed spectra for the different sources the same or different? How does your answer clarify the saying, "Spectra are like fingerprints". Activity 2: Energy & Color The energy of light is inversely proportional to the wavelength: E 1/ . Specifically, for distances measured in units of 10-5 cm (as recorded on your drawings) the energy should be given as E = 12.4/ . (The units of energy here turn out to be in "eV". If you know about eV, good for you; if not don’t worry about it) For the hydrogen spectrum you observed, fill in the table showing the color, the wavelength, and the energy for each of the lines you observed. (NOTE: If you did not have a clean hydrogen spectra, that is a series of lines, the values are: Red 6.55x10-5 cm; Blue 4.85 x 10-5 cm, Violet 1 4.35 x10-5 cm; Violet 2 4.10 x 10-5 cm) The energy levels for hydrogen can be predicted theoretically. If the lowest orbit is assigned an energy of 0, the higher orbits will be: 13.22eV 13.06eV 6 5 12.75eV 4 12.09eV 3 10.20eV 2 So I measure an emission line with a wavelength () of 4.85x10-5 cm. Using the equation, Energy=12.4E-5 / (10-5 cancels out), I can find my energy to be 12.4 / 4.85 = 2.55eV. An electron jumping down from the 4th orbit to the 2nd orbit would release light with an energy of (12.75 eV – 10.20 eV) = 2.55 eV. See if you can identify the orbits involved for the other lines. 12.75 – 10.20 = 2.55eV 10.20 – 0.00 = 10.20eV 0.00eV 1 Color Wavelength (10-5 cm) Energy (eV) Red Blue Violet 1 Violet 2 Questions: 1. Which has more energy, blue light or red light? How do you know? 2. What energy would you expect for orange light? Orbit Jump (start-end) Follow-up Questions 1. When an object is heated and starts glowing, it first appears a dull red. As it gets hotter, it turns orange, then yellow. Explain why this is logical in terms of your understanding of color and energy as it applies to a candle or electric stove. 2. Astronomers are interested in learning about the composition of stars and interstellar gas clouds. a.) Explain how this could be accomplished without having to actually visit the distant stars. b.) What advantages would there be in doing these experiments on Mars? 3. Ultraviolet light produced by the sun can be dangerous. Fortunately, very little of this UV light reaches the earth’s surface. What stops the UV light and what would be some problems for astronauts or Mars colonists?