Document
advertisement

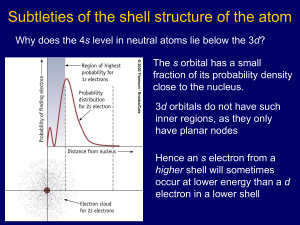
Do Now Write down anything about which you are unclear for the following topics: Atomic Theory Nuclear Chemistry Electron configurations Unit 2 Review Day Williamsburg High School for Architecture and Design Mr. Quinn and Ms. Tom 11/7/13 Aim How is the atom constructed? Atomic Theory Overview Plum Pudding Model Electrons Rutherford Model Nucleus Bohr Model Orbits Wave Mechanical Model Orbitals Check Yo’ Self, Part 1 What particles are located inside the nucleus? What is the sign of the charge of a nucleus? What is the charge of a proton? What is the mass of a proton and neutron? What is the sign of a charge on an electron? The Nucleus: an Overview Atomic Number Atomic mass Isotopes Nomenclature Number of protons Number of protons + neutrons Same protons, different neutrons Check Yo’ Self, Part 2 How many electrons, protons, and neutrons does a Kr-85 atom have? Which isotopic notation represents an atom of carbon-14? 1. 6 8C 2. 8 6C 3. 6 14C 4. 14 6C Nuclear Chemistry Half-Life Definition The time for half of a radioactive sample to decay Equation Nuclear Decay Equations Check Yo’ Self, Part 3 If an object has decayed for 3 half-lives, what fraction of the original remains? Balance the following nuclear decay equation: _________________→ 42He + 20682Pb Electron Configurations Bohr Model: shells Wave Mechanical model: orbitals Check Yo’ Self, Part 4 Part 1 A bromine atom in an excited state could have an electron configuration of 1. 2-8-18-6 2. 2-8-18-7 3. 2-8-17-7 4. 2-8-17-8 Identify 2 gases in the mixture Check Yo’ Self, Part 4 Part 2 Write the electron configuration for phosphorus in the ground state using orbital and electron spin notation. Now, it’s your turn!