Atomic Theorists Table
advertisement

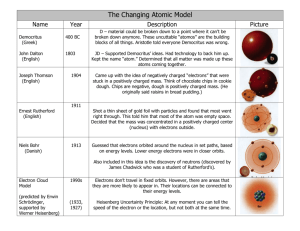
Scientist Democritus “MC Hammer” Dalton Model Greek Model Date 420 BC Discovered/Ideas Documented the idea of existence atoms Model Description solid sphere Billiard Ball Model 1800 Solid sphere Thomson Plum Pudding Model 1897 Proposed atomic theory Said that atoms make elements and elements combine to make compounds Discovered the electron Rutherford Nuclear model/ Planetary Model 1911 Bohr Planetary Model 1913 Schrodinger Electron Cloud Model or Quantum Mechanical Model 1924 Chadwick Neutron Model 1932 Through the “gold foil experiment” discovered that atoms have to be made up of mostly empty space, dense, positively charged region in the center that had to be small compared to the total size of the atom. proposed that the electrons orbit in distinct orbits, but electrons could jump between these orbitals by either gaining energy (moving to farther away electron shells), or by losing energy (falling to electron shells closer to the nucleus) came to view the atom not as a nucleus surrounded by electrons moving in perfectly circular orbits, but that as electrons moving in waves around the nucleus. Instead of perfectly circular orbits, the electrons would be found in clouds of space around the nucleus. Discovered/proved the existence of neutrons a sphere filled with a positively charged fluid, and negatively charged particles a dense region in the center, orbited by electrons like the rings around the planet Saturn electrons orbited the nucleus in concentric circles electrons are found in clouds of space around the nucleus Positively charged protons and similarly sized neutrally charged neutrons exist inside the positively charged nucleus




![The electronic configuration of phosphorus is [Ne] 3s2 3p3](http://s3.studylib.net/store/data/008974852_1-8381577ce936fbfa611892c1a5f109cd-300x300.png)



