Some Useful Equations - Wichita State University
advertisement

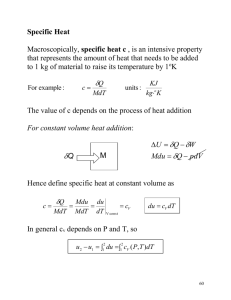
Conservation of Mass for a Control Volume Some Useful Equations Conservation of Energy Principle for a Closed System (the First Law of Thermodynamics) E = Q – W, where E = U + KE + PE + ... Conservation of Energy Principle for a Control Volume (z elevation) W > 0, work done by the system W < 0, work done on the system dE / dt]cv = Q – W + in m(h + V2/2 + gz) – out m(h + V2/2 + gz) [For transient (unsteady state) operations (i ≡ initial state, at time = 0 and f ≡ final state, at time = t) Work done by a closed system (mass = m) W = ∫ W = ∫ p dV = m ∫ p dv, where p = p(v) In a polytropic process = const.): p = (const.) / If n ≠ 1, W = (const.) (V21 – n – V11 – n)/(1 – n) = (p2V2 – p1V1)/(1 – n) If n = 1, W = (const.) ln V2/V1 mass flow rate (through an area A, with an average velocity V) m = AV = AV/v, where = density = 1/v, v = specific volume Q > 0, heat transfer to the system Q < 0, heat loss from the system (pVn dm / dt]cv = in m – out m mcv, f – mcv, i = in ∫ m dt – out ∫ m dt Vn Ucv, f – Ucv, i = ∫ Q dt – ∫ W dt + in ∫ mh dt – out ∫ mh dt with Ucv(t) = mcv(t) ucv(t), neglecting KE ≈ PE ≈ 0] The thermodynamic (Kelvin) temperature scale For a power cycle ( ʃ dU = 0) Wcycle = Qcycle = Qin – Qout > 0; efficiency, = Wcycle / Qin For refrigeration or heat pump cycles ( ʃ dU = 0) Wcycle = Qout – Qin < 0; CoP, = |Qsought | / |Wcycle| h = u + pv, or H = U + pV, where H = mh, U = mu, V = mv, etc. Two-Phase (liquid-vapor) mixtures Quality, x = (mvap / m) = mvap / (mliq + mvap) Any property (per unit mass), , of a liquid-vapor mixture: = (1 – x)f + x gf + x (g – f) For subcooled or compressed liquids: v(p, T) ≈ vf(T); u(p, T) ≈ uf(T); h(p, T) ≈ hf(T) The Compressibility Factor Z = pv / RT , where R = gas constant, = R / M, R = 8.314 kJ/kmol·K, M = molecular weight (kg/kmol) (in Figures A-1, A-2, & A-3: pR = p / pc, TR = T / Tc) dS = (Q / T)int rev ( Qint rev = ∫ T ds ) The first and the second “T dS” equations For ideal gases (per unit mass) ds = du / T + (R / v) dv = (cv / T) dT + (R / v) dv ds = dh / T – (R / p) dp = (cp / T) dT – (R / p) dp [for air, Table A-22, s(T2, p2) – s(T1, p1) = so(T2) – so(T1) – R ln (p2 / p1)] Isentropic processes (ds = 0) for ideal gases with a constant specific heat ratio, cp / cv = k T2 / T1 = (p2 / p1)(k – 1)/k ; pvk = p1v1k = p2v2k = const. p2 / p1 = pr2 / pr1 (with air, Table A-22) Entropy balance for a closed system with its boundary at Tb S2 – S1 = ∫ (Q / T)b + ( 0 ) Entropy rate balance for a control volume dS / dt]cv = (Q / T)b + in m s – out m s + cv Specific Heats constant volume specific heat, cv = ( u/ T)v constant pressure specific heat, cp = ( h/ T)p For an ideal gas pV = mRT) u2 – u1 = ∫ cv dT, where cv = cv(T) h2 – h1 = ∫ cp dT, where cp = cp(T) cp = cv + R For liquids and solids (incompressible substances, ≈ constant) cp ≈ cv = c, and H ≈ U = m c T ia:02/04 Entropy (defined in terms of a change in) T dS = dU + p dV T dS = dH – V dp Enthalpy (Z 1) pv = RT (or, u = u(T), cv = du/dT, h = h(T), cp = dh/dT, h = u + pv = u + RT; TC / TH = (QC / QH) for Carnot cycles max = 1 – (TC / TH ) Other Constants and Definitions Force, Work (Energy), and Power (rate of work, or heat transfer) 1 N = 1 kg·m/s2; 1 J = 1 N·m; 1 W = 1 J/s Pressure 1 Pa = 1 N/m2; 1 bar = 100 kPa (= 105 Pa = 0.1 MPa) 1 atm = 101.325 kPa = 1.013 25 bar (standard atmosphere) p (gage) = p (absolute) – p (atmospheric, absolute) Temperature T (ºC) = T (K) – 273.15 Standard Acceleration due to the Earth’s gravity g = 9.806 65 m/s2 SI Unit prefixes for multiplication factors c ≡ centi = 10–2; m ≡ milli = 10–3; ≡ micro = 10–6 k ≡ kilo = 103; M ≡ mega = 106; G ≡ giga = 109