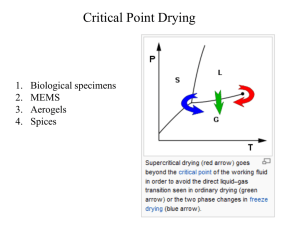
Specific Heat: Thermodynamics Lecture Notes
advertisement

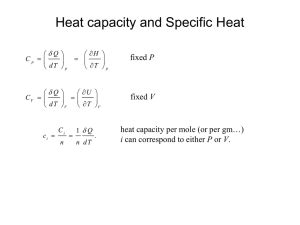
Specific Heat Macroscopically, specific heat c , is an intensive property that represents the amount of heat that needs to be added to 1 kg of material to raise its temperature by 1oK c For example : Q units : MdT KJ kgo K The value of c depends on the process of heat addition For constant volume heat addition: Q U Q W Mdu Q pdV M Hence define specific heat at constant volume as c Q MdT Mdu du MdT dT cV du cV dT V const In general cv depends on P and T, so u 2 u1 1 du 1 cV ( P, T )dT 2 2 60 For constant pressure heat addition: Q M U Q W Mdu Q pdV Mdu Q p ( Mdv) Q M (du Pdv) Define new property, enthalpy h h u Pv dh du Pdv vdP For constant P process dP=0, so dh = du + Pdv Substituting into the definition for c c Q MdT M (du Pdv) dh Mdt dT cP dh cP dT P const In general cP also depends on P and T, so h2 h1 12 dh 12 c P ( P, T )dT 61 Ideal Gas Assumption For an ideal gas cV and cP only vary with T, not P u 2 u1 12 cv (T )dT h2 h1 12 c P (T )dT Need absolute u and h. Set T2 to T and T1 to Tref h2 h1 h(T ) h(Tref ) TT c P (T )dT ref Take Tref =0 (absolute zero temperature) where h=0 h(T ) 0Tc P (T )dT h (T ) 0Tc P (T )dT for an ideal gas pv RT h u pv u RT u h RT u (T ) 0Tc P (T )dT RT u (T ) 0Tc P (T )dT R T cp values are tabulated in Table A-20 and Table A-21 gives an empirical relation for (c p / R ) as a function of T for various gases. 62 Enthalpy and Internal energy as a function of T for air is given in Table A-22, and for other gases in A-23. Get cV from cP differentiate h = u + RT with respect to T dh du R c P (T ) cV (T ) R dT dT dh du R c P (T ) cV (T ) R dT dT Note : Since R > 0 and cV > 0 cp > cV The specific heat ratio k is commonly used, for ideal gas k (T ) cP cV since c P cV k 1 see Table A - 20 For a substance with molar mass if you know k at a specific temperature can get cp and cV c P (T ) cV (T ) R c P (T ) cV (T ) R c P (T ) R 1 cV (T ) cV (T ) c P (T ) cV (T ) R K (T ) 1 R R K (T ) 1 K (T ) R c P (T ) K (T ) 1 63 Example: A tank contains 0.042 m3 of oxygen (O2) at 21C and 15 MPa. Determine the mass of the oxygen, in kg, using a) the compressibility chart b) ideal gas model Comment on the applicability of the ideal gas model O2 V= 0.042 m3 T= 21 C (294 K) P= 15 MPa (150 bar) a) From table A-1for oxygen: molar mass = 32 kg/kmol, Tc= 126K, Pc=50.5 bar PR P 150 bar T 294 K 2.97 ; TR 1.91 Pc 50.5 bar Tc 126 K From the Generalized Compressibility Chart Z 0.92 ZRT 0.92(8314/32 J/kg K)(294K) 3 v 0.0047 kg/m P 150x105 N/m 2 V 0.042 m 3 M 8.94 kg 3 v 0.0047 /m /kg 64 b) Assuming ideal gas PV (150x105 N/m 2 )(0.042m 3 ) M 8.24 kg RT (8314/32 J/kg K)(294K) Assuming the mass obtained from the chart is correct, the ideal gas model under predicts the mass by about 8%, not bad! Do the same problem with carbon dioxide (CO2): a) PR P 150 bar T 294 K 2.03 ; TR 0.97 Pc 73.9 bar Tc 304 K From the Generalized Compressibility Chart Z 0.3 v = 0.0011 m3/kg M = 38.2 kg b) Mideal = 11.34 kg Assuming the mass obtained from the chart is correct, the ideal gas model under predicts the mass by about 70%, bad! 65 Example: A piston-cylinder assembly contains 1 kg of nitrogen gas (N2). The gas expands from an initial state where T1= 700K and P1= 5 bar to a final state where P2= 2 bar. During the process the pressure and specific volume are related by Pv1.3= const. Assuming ideal gas behaviour and neglecting KE and PE effects, determine the heat transfer during the process, in KJ. P 1 Q Pv1.3= const N2 2 P1= 5 bar P2= 2 bar T1= 700K v U Q W Q U W W 12 pdV P2V2 P1V1 1 n Recall, for an ideal gas PV MRT 2 1 MR(T2 -T1 ) pdV 1 n need T2 66 R n 1 n P1v1 Pv P const P const 2 2 1 1 / n 2 1 / n T1 T2 T1 P1 T2 P2 n 1 n 2 P1 P T1 T2 T2 P2 T1 P1 n 1 n v v n 1 n 2 n 1 n T2 v1 T1 v2 n 1 0.3 1.3 2 bar T2 (700K) 567K 5 bar MR(T2 -T1 ) W 1 n 1 kg(8.314/28 kJ/kg K)(567 - 700)K 132kJ 1 1.3 Note: work is positive work done by the system The molar internal energy for nitrogen from Table A-23: u (700K) 14,784 kJ/kmol u (567K) 11,858 kJ/kmol 67 u u U M (u 2 u1 ) M 2 1 11,858- 14,784 kJ/kmol 1 kg 104.5 kJ 28 kg/kmol Q= U + W = (-104.5 kJ) + (132 kJ)= +27.5 kJ Note: heat transfer is positive heat transferred into the system 68 Constant Specific Heat Assumption This assumption can be made if the specific heat does not vary much in the temperature range of interest, use average values c~V and c~P such that: u 2 u1 TT cV (T )dT c~V TT dT c~V (T2 T1 ) h h T c (T )dT c~ T dT c~ (T T ) 2 2 1 1 2 2 1 T1 2 p P T1 P 2 1 the average value is taken to be c (T ) cV (T2 ) c~V V 1 2 c (T ) c P (T2 ) c~P P 1 2 Example: Recalculate U in last example assuming constant specific heat The specific heats for nitrogen from Table A-20 for the two temperatures are cV (700K) 0.801kJ/kg K cV (567K) 0.771kJ/kg K 69 The average value over the temperature range is thus (0.801 0.771) ~ cV 0.786 kJ/kg K 2 U M (u u ) Mc~ (T T ) 2 1 V 2 1 1 kg (0.786kJ/kg K)(567 - 700)K - 104.5 kJ The value for U obtained using Table A-23 which takes into account the change in cV with temperature is exactly the same!! If tables are not available can approximate cV by using the specific heat ratio K, which in the temperature range of 300K to 1000K, is taken to be constant (see Table A-20): Diatomic molecules K= 7/5= 1.4 (N2, O2, H2, CO, “Air”,…) Monatomic molecules (Ar, He, Ne,…) cV K=5/3=1.67 R 8.314/28kJ/kg K 0.742 kJ/kg K K 1 1.4 - 1 compared to 0.786 kJ/kgK calculated above 70 Incompressible Assumption A substance is incompressible if there is negligible change in specific volume with change in pressure, e.g., liquids and solids The specific internal energy of an incompressible substance does not vary much with pressure, therefore the specific heat only depends on temperature cV (T ) du(T ) dT v const By definition enthalpy varies both with P and T h(P,T) = u(T) + Pv For incompressible v is constant, differentiating with respect to T while maintaining P constant yields, dh(T ) du(T ) dT P const dT v const cP = cv = c 71 u 2 u1 12 c(T )dT Also, Δh Δ(u Pv ) h2 h1 (u 2 u1 ) ( P2 v2 P1v1 ) h2 h1 12 c(T )dT v( P2 P1 ) Two special cases: i) Constant pressure heat addition into an incompressible substance (P= const.) h2 h1 12 c(T )dT ii) Isothermal heat addition into an incompressible substance (T= const.) h2 h1 v( P2 P1 ) 72 Approximating enthalpy in the compressed liquid region of steam tables Want to get enthalpy at (P*, T*) without using compressible liquid tables Liquid at (P*,T*) Liquid at (P*,T*) Saturation line P* isobar T* P* isobar T1=T* P1(= Psat(T*)) isobar Saturation state (P1,T1) Consider the isothermal process (incompressible) from (P1,T1) (P*,T*) we now know h*- h1=v(P*- P1) h* h1 v( P * P1 ) h( P*, T *) h f sat (T *) v f sat (T *)( P * Psat (T *)) This gives a more accurate result than just taking h( P*, T *) h f sat (T *) as proposed earlier for u(P*,T*) The last term is a correction which is normally small because vfsat is very small 73