CHE 3290
advertisement

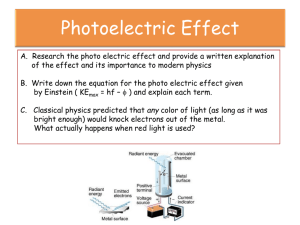
CHE 3200 THE PHOTOELECTRIC EFFECT SET-UP A 2015 OBJECTIVE: (a) Determine Planck’s constant h. (b) Determine the error in your value of h and compare your value with the literature value. (c) Determine the work function w for the particular metal used for the cathode of the vacuum tube of the h/e apparatus. (d) Determine the error in w. (e) Verify that the INTENSITY (amplitude) of the radiation is proportiona to the NUMBER of photons of the same energy h . (f) Estimate the number of electrons hitting the anode per second for a particular frequency and for several intensities of the electromagnetic radiation shined on the metal cathode. BACKGROUND: Read : Chapter 1 of James E. House, Fundamentals of Quantum Chemistry Planck’s formula : E = h (1) was used by Einstein to explain the so called Photoelectric Effect. = frequency of a photon E = energy of one photon h = proportionality factor , called Planck’s constant by Einstein Light consists of “light particles” called photons. Light of frequency consists of a stream of photons of which each one has energy h and travels with a speed c (speed of light). Light of another frequency consists of photons of a different energy, namely h which travel with the same speed c. The intensity of light of frequency depends on the number of photons of energy h . Higher intensity means MORE photons. Each photon removes ONE electron. It takes a certain minimum amount of energy to remove one electron from a metal surface. This energy depends on the metal and is called ‘work function’ w. One photon can remove one electron from a metal surface when the energy of the photon is equal or greater than w. When the energy of the photon is greater than w the difference in the energy is given to the electron as kinetic energy. The photon which can be thought of as a bundle of energy (h ) ceases to exist after the process. All of its energy is negotiated to the electron, not just a part of it. Note : w is the minimum energy needed – this means that some electrons need more energy, but no electron needs less. Therefore: Ek = h - w (2) Ek = kinetic energy of the electron (this is the maximum energy an electron can have) Since each photon can remove one electron from the metal surface, the number of electrons is proportional to the INTENSITY of the light shining on the metal. A1