Photoelectric Effect*Post-Lab Summary
advertisement

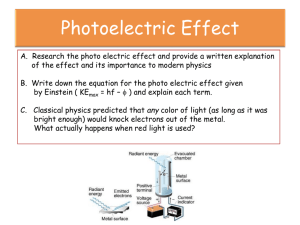
Photoelectric Effect— Post-Lab Summary Photon Energy • Energy of Photons is dependent on the frequency of the light. • Reminders: – Speed of light (c) = 3.00 x 108 m·s-1 𝑐 = 𝑓𝜆 Energy of a Photon: 𝐸 = ℎ𝑓 Practice Problem • A beam of blue light (wavelength = 455. nm) is emitted from a light source. – What is the frequency of this light? 8 𝑐 3.00𝑥10 14 𝑓= = = 6.59𝑥10 𝐻𝑧 −7 𝜆 4.55𝑥10 – What is the energy of the blue photons? 𝐸 = ℎ𝑓 = 6.626𝑥10−34 6.59𝑥1014 𝐸 = 4.37𝑥10−19 𝐽 1 𝑒𝑉 −19 𝐸 = 4.37𝑥10 𝐽 = 2.73 𝑒𝑉 −19 1.60𝑥10 𝐽 What usually happens as energy is absorbed? • Classical Explanation: – a substance will continue to gain energy every time energy is transferred to it – For example, if a child on a swing is given a push every time he reaches the peak of his swing, he will continually gain energy from each subsequent push, and will steadily swing higher and higher. – If one push isn’t enough to make him reach a certain height, eventually he’ll get there. (It might just take a while) Does this work for electrons in a metal when energy is added? • In a word, NO. • Electrons must gain a certain amount of energy in order to break completely free from the surrounding atoms. • This amount of energy must come at once, not over a period of time Energy gained by electrons • When photons hit a metallic surface, the energy from the photon is absorbed by an electron • IF there isn’t enough energy in the single photon to make the electron break free, it won’t. Simple as that. • If there, is, then it will. Simple as that. Threshold Frequency (Cutoff frequency) • The frequency that carries the minimum amount of energy necessary for an electron to absorb in order for it to be emitted from the surface of a metal Work Function (ϕ) • The energy, in eV, that must be absorbed by an electron in order for it to be emitted from the surface of a metal • Work Function is dependent on the type of metallic surface. Not its size, or temperature or anything else—just the type. Practice Problem #2 • The work function for a piece of Aluminum is 4.08 eV. • How much energy, in Joules, must an electron absorb in order to be emitted from the surface of aluminum? • What frequency of light would allow this to occur? • Where in the spectrum is this light? What about intensity of light? • Does intensity affect the amount of energy an electron absorbs? • NO! Each photon of light can eject a single electron. So if there is a higher intensity, that means there are more photons. More photons = more electrons…but not more energy per electron. What if a photon has more energy than the electron needs? • Additional energy, over and above the work function, that is supplied by the photon will be converted into the kinetic energy of the electron Graphical Representation Work Function • What does the slope represent? Graphical Representation Work Function 𝑬𝒌,𝒎𝒂𝒙 = 𝒉𝒇 − 𝝓 Stopping Voltage • When a piece of metal is subjected to light and produces a photocurrent, the stopping voltage is the amount of potential that must be added in order to just stop the forward motion of the electrons. • The voltage that just brings the current down to zero (0) Stopping Voltage • What must be done to stop an electron? – A force must be applied; work must be done • What must change when the electron comes to a stop? – Kinetic energy of the electron reaches 0 • How do we represent that mathematically? 𝑾 = 𝒒𝑽 𝑬𝒌 = 𝒆 ∙ 𝑽 Assume that a 180 W lightbulb gives off 2.80% of its energy as visible light. How many photons of visible light are given off in 2.90 minutes? (Use an average visible wavelength of 550 nm.) A metal with a work function of 2.30 eV is illuminated with a beam of monochromatic light. It is found that the stopping potential for the emitted electrons is 2.30 V. What is the wavelength of the light in nanometers? The work function of a photoelectric material is 3.5 eV. Assume the material is illuminated with monochromatic light (λ =250 nm). (a) What is the stopping potential of the emitted photoelectrons? (b) What is the cutoff frequency?