Chemical Bonding Lab Purpose: To use physical properties to
advertisement

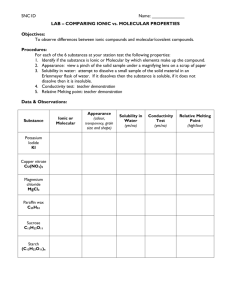
Chemical Bonding Lab Purpose: To use physical properties to distinguish between ionic and molecular compounds. Introduction: Chemical compounds are combinations of atoms held together by chemical bonds. These chemical bonds are of two basic types - ionic and covalent. Ionic bonds result when one or more electrons from one atom are transferred to another atom. Positive and negative ions are created. In covalent bonds, the electrons are shared by the bonded atoms. Ionic compounds are composed of large numbers of cations and anions so that the amount of positive charge equals the negative charge. Molecular compounds are composed of covalently bonded molecules. The physical properties of a substance such as melting point, solubility in water and conductivity of an aqueous solution tell us a lot about the type of particles in a compound. Properties of compounds depend on the strength of the attractive forces between particles. The particles that compose an ionic compound (ions) are held together by ionic bonds. The particles that make up a molecular compound (molecule) are held together by intermolecular forces. In this experiment, you will conduct tests on the physical properties of different compounds and compile data enabling you to classify the compounds as ionic or molecular. Pre Lab instructions A. Title B. Purpose C. Answer the following in complete sentences 1. Define ion 2. Define molecule 3. What particles are in an ionic compound? What forces hold them together? 4. What particles are in a molecular compound? What forces hold them together? 5. Define electrolyte 6. Why is water referred to as the universal solvent? D. Write out procedures in your own words E. Copy or print out data table Procedures Test 1. Analyzing Appearance In separate aluminum weighing dishes, collect half of one scoop of each of the following chemicals: potassium chloride, dextrose, citric acid, sodium sulfate, sodium carbonate, and phenyl salicylate. Label the dishes (Aluminum foil) using a permanent marker. 1. What does the substance look like to the naked eye? 2. What does the substance look like when observed using a hand lens? 3. For each substance, identify the color, texture, particle size, particle shape, and any other physical properties that you can. Record in your table. Test 2. Determining Melting Point For this test, write the numbers 1-6 on the aluminum foil. Place the aluminum foil on the ring (fold the edges down so it will not fall off). 1. Set up the Bunsen burner. Be sure that the ring is positioned on the ring stand so that it is above where the tip of the flame will be. 2. Place a few crystals of each substance on to the aluminum foil next to its number (from table). 3. Light the burner. Adjust the flame so that the air vent is completely closed and the flame is low (flame may be yellow). Make sure the flame does not touch the foil! You are melting NOT burning! Record the order of melting in your data table (1st, 2nd, etc…). After ten minutes, record an N in your data table for each substance that did not melt. [Note: If the compound does not melt, the temperature was not hot enough to reach the melting point; melting point must be relatively high.] 4. Extinguish the flame and allow the aluminum dish to cool while you complete the rest of this experiment. Test 3. Determining Solubility 1. Place a few crystals of each of the white solids in wells 1-6 in the well plate. Make sure you use the numbers from the table 2. Add 10 drops of distilled water to each of the six compounds. 3. Stir gently using toothpick. (Use a different toothpick for each one). It is necessary to observe the solids for several minutes to determine solubility. 4. If the chemical dissolves, record “yes” in the table. If it does not dissolve, record “no.” 5. Do not dump your chemicals. You will use these solutions for your next Test. (Test 4) Test 4. Determining Conductivity 1. Create a setup as demonstrated by your teacher using a D-cell battery, a battery holder, one LED, paper clips, and connecting wires with alligator clips. a) Connect a wire from the negative terminal of the battery to the short end of the LED. b) From the long end of the LED, connect a second connecting wire with a paper clip on the end. c) From the positive terminal of the battery, connect the third connecting wire with a paper clip on the end. d) Test the LED by touching the paper clips together and watching for light. 2. Test the conductivity of the solutions you created during the solubility test by placing the ends of each paper clip into the solution. Be sure both clips are in the solution, but do not let them touch each other. Rinse and dry the paper clips between each of the tests. 3. If the substance is a strong conductor of electricity, write “Strong” in the appropriate cell in the table. If the substance is a weak conductor of electricity, write “Weak” in the appropriate cell in the table. If the substance is not a conductor, write “No.” Disposal and cleanup: Clean the well plate using brush and water. Turn upside down on a paper towel. Throw away aluminum foil. Wipe down your area. 1 Chemical Potassium Chloride 2 Dextrose 3 Citric Acid 4 Sodium Sulfate 5 Sodium Carbonate 6 Phenyl Salicylate Bond Type Appearance Melting Point Solubility Conductivity Post Lab Analysis: 1. Group the compounds into two groups according to their properties. List the properties of each group. Include melting point (high or low), conductivity of an aqueous solution, and solubility in water. Ex. Group one consists of (name compounds). They generally have (discuss properties). Group two consists of (name compounds). They generally have (discuss properties). 2. Do all of the members of the group exhibit all of the properties? Give specific examples from this experiment. Conclusions: 1. Determine which of the groups consists of ionic compounds and which consists of molecular compounds. a. Write a general statement to summarize the properties of ionic compounds. Include state at room temperature, melting pint, conductivity of aqueous solution, and solubility in water. b. Write another general statement to summarize the properties of molecular compounds. Include state at room temperature, melting pint, conductivity of aqueous solution, and solubility in water. 2. How is melting point related to the strength of attractive forces between the particles of a compound? Which are stronger, ionic bonds or intermolecular forces? Which have higher melting points, ionic compounds or molecular compounds? 3. Compare and contrast the appearance of covalent and ionic compounds. When observing the substances with the hand lens, what properties do you notice that you could not see with your naked eye? 4. Imagine looking at the substances under a microscope. What do you think the substances might look like on that level?