1 temp. - ChemConnections
advertisement

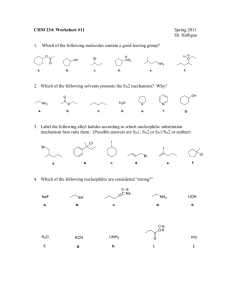
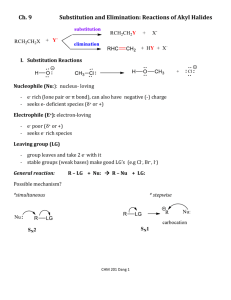
Name: _________________ Chem 226 / Fall 2012 Lab Section: Circle one. MW(11:00) or MW(2:30) Answer questions #1-#30 on the Scantron. #1-20, 4pts each; #21-30, 2pts each; answer remaining questions on the exam [pt. values indicated]; [Exam = 130 possible raw pts total] 1. Which compound can have four stereoisomers? (Be sure to consider E- & Z- diastereomers.) A) B) C) D) 5-bromo-2,4-dimethyl pentene 5-bromo-3,4-dimethyl-2-pentene 1-bromo-2,4-dimethyl-2-pentene 2-bromo-3,4-dimethyl pentene 2. A reaction mixture consisted of 0.5M methyl iodide and 1.0 M sodium hydroxide. The measured rate of reaction at room temperature was 0.05 mol/L per second. What would be the predicted rate for a reaction mixture of 1.0 M methyl iodide and 2.0 M sodium hydroxide? A. B. C. D. E. 0.05 mol/L per second 0.20 mol/L per second 0.10 mol/L per second 0.25 mol/L per second 0.50 mol/L per second 3. The most stable chair conformation of cis-1-tert-butyl-2-methylcyclohexane has A. B. C. D. both groups equatorial. both groups axial. the tert-butyl group equatorial and the methyl group axial. the tert-butyl group axial and the methyl group equatorial. 4. Consider the molecule below. What is the maximum number of methyl groups that can be in the equatorial position at the same time? A. 0 B. 1 C. 2 D. 3 E. 4 5. In the dehydrohalogenation of 2-bromobutane, which conformation below leads directly to the formation of cis2-butene? A. B. C. D. E. only I only II only III I and II none 6. Which of the following cannot undergo an E2 reaction? A. B. C. D. E. only I only II only III I and III none 7. Consider the SN1, SN2, E1, and E2 mechanisms. The energy diagram shown below corresponds to A. B. C. D. only the SN1 mechanism. only the SN2 mechanism. both the SN1 and E1 mechanisms. both the SN2 and E2 mechanisms. 8. Consider the reaction coordinate/ energy diagram shown below. Which of the following statements best describe the reaction? A) B and D are Transition States; A and C are Intermediates; the rate determining step is the formation of A, which is less stable than C. B and D are Transition States; A and C are Intermediates; the rate determining step is the formation of B, which is less stable than D. C) A and C are Transition States; B and D are Intermediates; the rate determining step is the formation of D, which is more stable than B. D) A and C are Transition States; B and D are Intermediates; the rate determining step is the formation of B, which is less stable than D. B) 9. Which of the following molecules are chiral? A. B. C. D. E. only II only III II and III I, II, and III none 10. How many of the following three molecules respectively have chiral centers, and how many are actually chiral molecules? HO HO HO H O C OH C H C OH OH C OH OH C C C OH C OH O H C F H Cl O C H C H C C O OH OH OH OH A. 3, 3 B. 3, 2 C. 2, 2 D. 2, 1 E. 3, 1 11. A pure sample of an amino acid (+)-(S)-phenylalanine, which has a specific rotation of +70o, is reacted with a racemic mixture of the analgesic ibuprofen to produce two diastereomeric salts, (+, +) and (+, -). The (+, -) C diastereomer has a specific rotation of +7.0. What are the percentages of the S- and R- enantiomers of ibuprofen contained in the (+, -) product? A. B. C. D. E. 95% S, 5% R 90% S, 10% R 55% S, 45% R 52.5% S, 47.5% R Cannot determine 12. The following structures represent: A. same molecules B. enantiomers C. diastereomers D. different molecules E. constitutional isomers 13. The four isomeric dimethylcyclopropanes are shown below. Identify two of the isomers which are related as diastereomers. A. B. C. D. I and III I and IV II and III III and IV 14. Which of the following Fischer projections corresponds to the compound shown below? 15. Which of the following carbocations is (are) likely to undergo a rearrangement? A. B. C. D. only I I and III II and III I, II, and III 16. Rank the following from slowest to fastest for solvolysis in ethanol/water? A) II < III < I < IV B) II < III < IV < I C) I < IV < III < II D) I < II < III < IV 17. Which prediction is most likely correct for the ranking of the following solvent systems in decreasing order of reactivity under Sn1 reaction conditions? I) 50%:50% ethanol:water A. B. C. D. E. II) 50%:50% methanol:water III) water IV) 50%:50% acetone:water III > IV > II > I IV > III > II > I II > III > IV > I III > II > I > IV II > IV > III > I 18. Rank the following substrates in decreasing order of reactivity under Sn2 reaction conditions: I) methyl bromide A. B. C. D. E. II) 1-chloropropane III) 1-chloro-2,2-dimethylpropane IV) methyl iodide I > IV > II > III IV > III > II > I II > IV > I > III III > IV > II > I IV > I > II > III 19. Which one of the following descriptions correctly fits the substitution reaction below? (The product is a Fisher projection.) Likely mechanism Rate expression Stereochemistry 20. Which of the following does not give 1,2-dimethylcyclohexene as one of the acid-catalyzed dehydration products? End of Multiple Choice True (A)/ False (B): Answer on Scantron 21. If a molecule has only one stereogenic center it must be chiral. 22. The structures below have opposite optical rotations, one levo and one dextro. 23. An optically active (+)-R-alcohol isomerizes in acid to produce a mixture which has []20 = 0°. This is because all of the starting alcohol has been converted to the opposite enantiomer. 24. Bimolecular reactions have at 2 Transition States. 25. The following mesylates react with sodium iodide in acetone. The relative rates from the fastest to the slowest are: II > I > III > IV . 26. The following arrows and illustration show the correct electron movements and transition state’s stereochemical arrangement for an E2 reaction mechanism. 27. The biochemical conversion of norephedrine to adrenalin is similar to an Sn2 reaction. 28. In the following reaction, a kinetics experiment was conducted where a 25 mL solution, which had respectively 5 mmol of bromide and 5 mmol of sodium hydroxide, reacted in 15 sec. It is expected that if the volume of the solution were doubled and the # of moles kept the same, the reaction would take place in about 30 sec. 29. The slow, rate-determining step, in the acid-catalyzed dehydration of 2-methyl-2-propanol is the loss of water from the oxonium ion to form a carbocation. 30. The following Fisher structures are respectively R- and S- enantiomers. End of True/ False 31. [4pts] Only the (S)-enantiomer of the analgesic, ibuprofen is biologically active. Complete the perspective drawing for the active isomer and its Fisher drawing next to it. Using the Cahn-Ingold-Prelog priority system, number the substituent groups in the Fisher drawing: 1, 2, 3, 4; where 1 = highest and 4 = lowest priority. 32. [3pts] The following thermodynamic data were obtained for experimental oxidation of 3 different alkenes with the same mole cular formula. Match the structures to the data. Compound A) B) C) ____ (Z)-2-pentene Heat of Reaction (kJ/mol) -186 -164 -162 ____ 2-methyl-2-butene ____ (E)-2-pentene 33. [3pts] Circle the stronger nucleophile in each pair: NH3 vs. PH3 I- vs. Cl- CH3OH vs. CH3O- 34. [10pts] Complete the mechanism for the following reaction. Draw structures for the two intermediates. Use arrows for the electron movement to show each step in the formation of the alkene. Use water in the last step. Draw an energy diagram below illustrating the reaction: EaI = +25kJ; ΔHI = +10kJ; EaII = +10kJ; ΔHII = +5kJ ; EaIII = +5kJ; ΔHRxn= -25kJ 35. [10pts] Peramivir is a relatively new antiviral drug. The active stereoisomer and the IUPAC name are shown below. It was developed by BioCryst Pharmaceuticals in collaboration with Green Cross Pharmaceuticals in South Korea and Shionogi Pharmaceuticals in Japan. It is approved for treatment of H1N1 influenza by the U.S. Food and Drug Administration. * (+)(1S,2S,3S,4R)-3-[(1S)-1-acetamido-2-ethyl-butyl]-4-(diaminomethylideneamino)-2-hydroxycyclopentane-1-carboxylic acid; / []20 = +57°, c = 0.5, ethanol Peramivir is an enzyme inhibitor, acting as a competitive transition-state inhibitor of influenza neuraminidase and thereby preventing new viruses from emerging from infected cells. It is administered by injection and offers improved treatment over other antivirals, Tamiflu and Relenza, which are presently in use. How many stereocenters are present in peramivir? How many stereoisomers are possible? __________ __________ Identify three different functions in the molecule. (Name them below, circle and number them in the structure above.) 1) ____________________ 2) ____________________ 3) ____________________ What is the absolute configuration of the carbon to the left of the * in the structure? ___________ A stereospecific synthesis of peramivir, BCX-1812, (RWJ-270201), has been reported by synthetic chemists affiliated with Green Cross/Shionogi, who have friends and associates currently studying Chem 226 topics on-line. The synthesis reported an overall yield of 60% and an optical yield of 80% of the biologically active isomer. What is the relative configuration of the biologically active stereoisomer? (circle one): d- or lWhat is the enantiomeric excess of the main stereoisomer of peramivir in the synthesized sample? ________ Which stereoisomer is in excess? (circle one): d- or lWhat is the % of the biologically active stereoisomer present in the sample? _________