In-class exam of 3/16/99 covering Chapters 6-8
advertisement
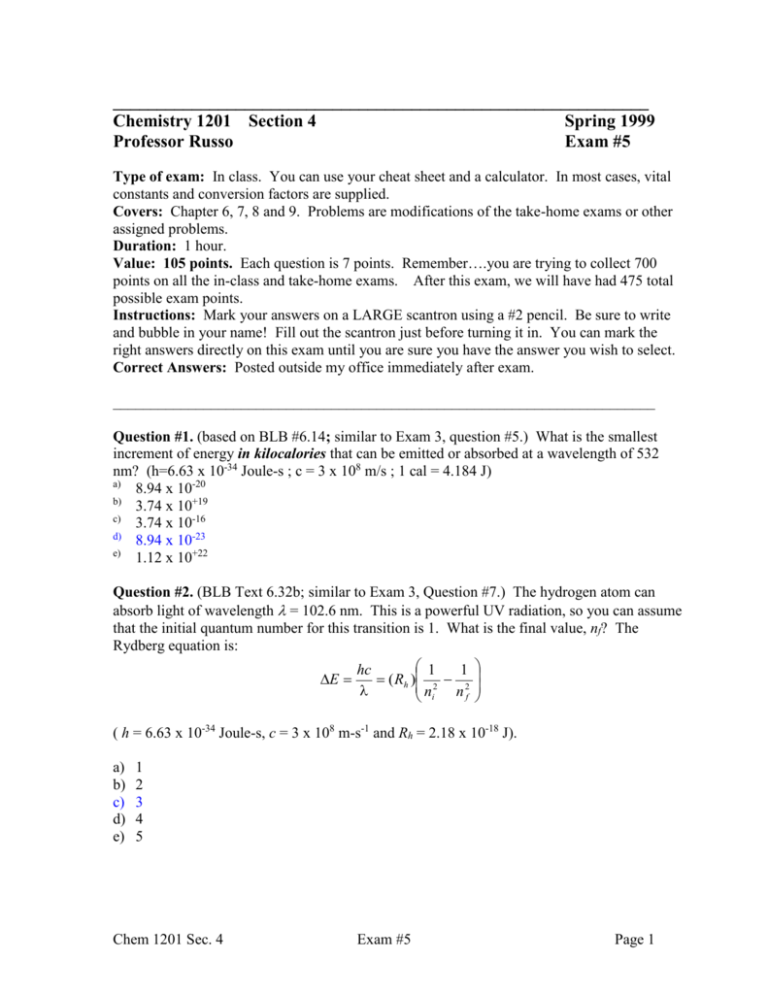
_____________________________________________________________ Chemistry 1201 Section 4 Spring 1999 Professor Russo Exam #5 Type of exam: In class. You can use your cheat sheet and a calculator. In most cases, vital constants and conversion factors are supplied. Covers: Chapter 6, 7, 8 and 9. Problems are modifications of the take-home exams or other assigned problems. Duration: 1 hour. Value: 105 points. Each question is 7 points. Remember….you are trying to collect 700 points on all the in-class and take-home exams. After this exam, we will have had 475 total possible exam points. Instructions: Mark your answers on a LARGE scantron using a #2 pencil. Be sure to write and bubble in your name! Fill out the scantron just before turning it in. You can mark the right answers directly on this exam until you are sure you have the answer you wish to select. Correct Answers: Posted outside my office immediately after exam. ________________________________________________________________________ Question #1. (based on BLB #6.14; similar to Exam 3, question #5.) What is the smallest increment of energy in kilocalories that can be emitted or absorbed at a wavelength of 532 nm? (h=6.63 x 10-34 Joule-s ; c = 3 x 108 m/s ; 1 cal = 4.184 J) a) 8.94 x 10-20 b) 3.74 x 10+19 c) 3.74 x 10-16 d) 8.94 x 10-23 e) 1.12 x 10+22 Question #2. (BLB Text 6.32b; similar to Exam 3, Question #7.) The hydrogen atom can absorb light of wavelength = 102.6 nm. This is a powerful UV radiation, so you can assume that the initial quantum number for this transition is 1. What is the final value, nf? The Rydberg equation is: 1 hc 1 E ( Rh ) 2 2 n i nf ( h = 6.63 x 10-34 Joule-s, c = 3 x 108 m-s-1 and Rh = 2.18 x 10-18 J). a) b) c) d) e) 1 2 3 4 5 Chem 1201 Sec. 4 Exam #5 Page 1 Question #3. (similar to BLB 6.75, or Exam 3, Question #9). The most powerful laser in my lab produces 2.50 Watts at 532 nm. How many photons does it emit in one minute? Express your answer in mol. (Remember: 1 mol of something is 6.02 x 1023 and Power in Watts is given by energy in Joules divided by time in seconds: P = E/t. One Watt = 1 Joule per second.) a) 4.01 x 10+20 b) 6.67 x 10+5 c) 6.67 x 10-4 d) 1.11 x 10-5 Question #4. (based on 6.66) The correct electron configuration for Mn is best represented by: a) [Kr] b) c) d) 4s 3d 4s 3d [Ar] [Ar] 4s 3d 4s 3d [Ar] Question #5. (based on 8.88, assigned & worked in class) Draw the best structure for perchlorate anion, ClO4-1. For the central chlorine atom, the formal charge and oxidation numbers are, respectively: a) +3, +7 b) 0, +8 c) –2, +7 d) +2, +7 e) +5, -6 Question #6. (based on 8.64) True or False: ammonia has a tetrahedral electron symmetry, so it is a nonpolar molecule. a) True, ammonia has no polarity. b) False, ammonia is polar. Chem 1201 Sec. 4 Exam #5 Page 2 Question #7. (based on 9.19) Which of the following molecules are polar? I) CCl4 a) b) c) d) e) II) SO3 III) SF4 IV) NF3 V) PF5 I and V II only III and IV All of them None of them Question #8. (based on BLB 9.16) Give approximate values for the indicated C-C-N bond angle in the following molecule (it is not drawn in any kind of 3D perspective right now; time to use those electron domain concepts). H H C C N: H a) b) c) d) 90o 109.5o 120o 180o Question #9. (based on BLB 9.16) Give approximate values for the indicated H-C-C bond angle in the following molecule. H H a) b) c) d) 90o 109.5o 120o 180o C C N: H A carbon with 4 single bonds is tetrahedral Question #10. (based on BLB 6.34) A helium atom (6.65 x 10-24 g) moving at a speed of 8.5 x 105 m/s has what deBroglie wavelength? (Remember: h = 6.63 x 10-34 Joule-s and 1 Joule = 1 kg-m2-s-2) a) 8.52 x 1015 m b) 1.17 x 10-16 m c) 3.33 x 10-19 m d) 1.93 x 1062 m e) 1.17 x 10-13 m Chem 1201 Sec. 4 Exam #5 Page 3 Question #11. (based on BLB 7.13) Arrange the following in order of increasing atomic radius: F, P, S, As. Put the largest atom on the right, smallest on the left. a) F < P < S < As b) As < P < S < F c) F < S < P < As d) As < S < P < F Question #12. (based on BLB 7.23) Which has the higher first ionization energy, Mg or Sr? a) Mg b) Sr Question #13. (based on BLB 7.23) Which has the higher first ionization energy, As or Br? a) As b) Br Question #14. (based on BLB 8.38) Referring only to the periodic table, arrange Ga, As, O in order of increasing electronegativity. (Most electronegative atom on the right). a) Ga < As < O b) O < As < Ga c) As < O < Ga d) O < Ga < As e) As< Ga < O Question #15. (BLB 8.79) Use formal charge arguments to decide which of the following resonance structures for N2O is the least important. .. .. .. .. a) : NN-O : b) :N-NO: c) :N=N=O: .. .. _____________________________________________________________________ There! You have survived two in-class exams. Things to note: The assigned problems re-appeared. If you are only working the take-home exams, you struggled. Only by working the assigned problems and the take-home exams can you work quickly enough to do well! In the very near future, you will not be sorry for working some additional homework problems. Chem 1201 Sec. 4 Exam #5 Page 4