ORGANIC CHEMISTRY – Naming of alkanes
advertisement

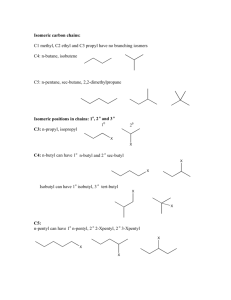
Date: Name: Class: ORGANIC CHEMISTRY – Naming of alkanes Answer the following questions on this sheet 1. Draw the skeletal formulae of all alkanes with the molecular formula C6 H14 and name the substances according to the IUPAC rules. (Elemente, p. 295, A3) n-hexane german: n-Hexan engl: 2-methylpentane 3-methylpentane 2,2-dimethylbutane 2,3-dimethylbutane 2-Methylpentan 3-Methylpentan 2,2-Dimethylbutan 2,3-Dimethylbutan 2. Draw the skeletal formulae of the following molecules. (Elemente, p. 297, A7) a) 3,3-dimethylhexane b) 2-methylhexane c) 3-ethylhexane d) 3-ethyl-2-methylpentane e) 2,3,4-trimethylhexane Which molecules are isomers? a), c) and d) are isomers of octane. 3. Some of the following names are not correct. Find out the right names and give an explanation. (Elemente, p. 297, A8) a) 1-methylhexane b) 2-ethylhexane c) 3-ethylhexane n-heptane A methyl group at pos 1 extends the chain d) 2-methylethane 2-methylheptane ok An ethyl group at position 2 extends the chain e) 3-propyl-2-methyl-3-ethylhexane n-propane A methyl group at the last C extends the chain f) 2,2-methylpropane 3-ethyl-2-methyl-3-propylhexane 2,2-dimethylpropane The side chains must be ordered alphabetically. In fact, the longest chain is -heptane, but -hexane is preferred as main name, because with -heptane a subbranch would occur. Two methyl groups need the prefix "di" 4. Name this molecules (Elemente, p. 297, A9) a) 3,3,5-trimethylheptane b) 3-ethyl-4-methylheptane c) 3,3,5,5-tetramethyloctane d) 3,5-diethyl-2-methyloctane 5. Which of the following formulae represent structural isomers and which ones are identical? (Elemente, p. 297, A10) structural isomers: identical: a, b, c, e, h: C5H12; d, f, g, i, j, k, l: C6H14 a, b, h: 2-methylbutane; Kantonsschule Kreuzlingen, Klaus Hensler d, l: 2-methylpentane; g, j: 2,2-dimethylbutane Che10_AlkanesNames_ARBBLATTengl.doc – 18.02.2010 1