SCH3U Chemistry Solutions Unit Review
advertisement

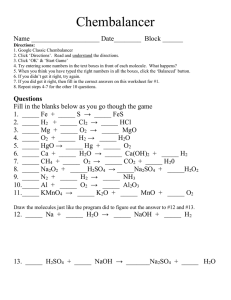
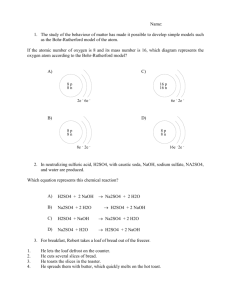
1. a) e) i) m) q) 2. 3. a) b) c) d) e) 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. 18. a) c) e) 19. 20. 21. SCH3U Solutions Unit Review Definitions : miscible b) immiscible (c) solute (d) solvent solution f) unsaturated (g) saturated (h) supersaturated pH j) acid (k) base (l) neutral neutralization n) amalgam (o) aqueous (p) electrolyte ionic r) covalent (s) titrant (t) endpoint What is the universal solvent and why? Make the following calculations and be sure to include the units in your answer: What is the w/w % of brass where there is 27 g of aluminum dissolved in 123 g of copper? What is the w/v % of a solution with 4.00g of sodium monoflourophosphate in 96.0 mL of water? What is the v/v% of a solution with 4.00 mL of acetic acid in 96.0 mL of water? What is the ppm of a solution that has 5mg of lead in 10 L? What is the ppb of a solution that has 35 mg of oxygen in 500 mL? What are the 2 different types of units for molar concentration? What is the molar concentration of a solution that has 730 g of hydrogen chloride dissolved in 10.0 mL of water? What mass of bromine gas can be found in 355 mL of a solution with a concentration of 2.54 M? What is the volume of a solution that contains 15.0 mol of sodium acetate and has a concentration of 2.00 M? What is the volume of concentrated sulfuric acid (18 M) that is required to make 7.00 L of 0.15 M H2SO4? What is the concentration of a diluted base if you have made 10.0 L from only 10.0 mL of 15 M NaOH? What is crystallization and what are 2 ways that it can occur? What makes an acid an acid and a base a base? What are 5 characteristics of acids (do not include indicators)? What are 5 characteristics of bases (do not include indicators)? What are 4 indicators and what does each do in acid, base and neutral solution? What is the pH of a solution of 2.70 x 10 -4 M hydrochloric acid? What is the hydronium ion (H3O+, same as H+) concentration of a pH 2.50 acid? What is a titration experiment and why is it important? Predict the products of the following reactions: NaOH + H2O (b) H2SO4 + H2O CH3COOH + H2O (d) NH3 + H2O H3PO4 + H2O Explain in your own words how to use a buret. Titration reveals that 11.6 mL of 3.0 M sulfuric acid are required to neutralize the sodium hydroxide in 250.0mL of NaOH solution. What is the molarity of the NaOH solution? If it takes 50 mL of 0.5 M KOH solution to completely neutralize 125 mL of sulfuric acid solution (H 2SO4), what is the concentration of the H2SO4 solution? a) what is the solubility of NaNO3 at 45 ºC? b) At what temperature are the two nitrates equally soluble? c) At 30 ºC what volume of water is required to dissolve 45.0 g of KI? d) what solute shows an inverse relationship between solubility and temperature? e) Shade the region of the graph where NaCl is unsaturated and KClO3 is supersaturated.