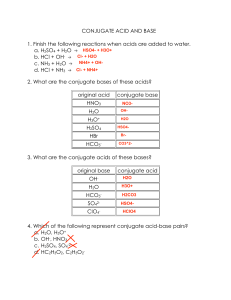
Homework 12 1. For the following reactions, identify the acid, base, conjugate acid and conjugate base: a. HF + H2O → H3O+ + Fb. CN- + H2O → HCN + CNc. HCN + NO2 → HNO2 + CN2. Write a balanced chemical equation to represent each of the following acid-base neutralization reactions a. H2SO4 and LiOH b. Ba(OH)2 and H3PO4 3. Complete the following table: [H3O+] pH 6.2 X 10-8 7.21 Acidic or Basic? Basic 7.2 X 10-8 5.30 1.50 X 10-3 4. Predict whether each of the following pairs of substances could function as a buffer system in aqueous solution: a. HNO3 and NaNO3 c. KCl and KCN b. HF and NaF d. H2CO3 and NaHCO3 5. Determine the molarity of a NaOH solution when each of the following amounts of acid neutralizes 25.0 mL of the NaOH solution: a. 5.00 mL of 0.250 M HNO3 b. 20.00 mL of 0.500 M H2SO4 c. 10.00 mL of 0.100 M H3PO4