grade 10 test 2
advertisement

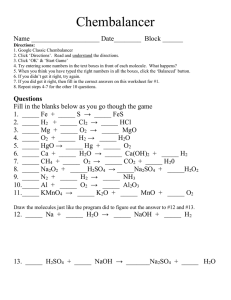
Name: 1. The study of the behaviour of matter has made it possible to develop simple models such as the Bohr-Rutherford model of the atom. If the atomic number of oxygen is 8 and its mass number is 16, which diagram represents the oxygen atom according to the Bohr-Rutherford model? A) C) 8p 8n 16 p 16 n 2e 6e 6e 2e B) D) 8p 8n 8p 8n 8e 2e 16e 2e 2. In neutralizing sulfuric acid, H2SO4, with caustic soda, NaOH, sodium sulfate, NA2SO4, and water are produced. Which equation represents this chemical reaction? Na2SO4 + 2 H2O A) H2SO4 + 2 NaOH B) Na2SO4 + 2 H2O C) H2SO4 + NaOH Na2SO4 + 2 H2O D) Na2SO4 + H2O H2SO4 + 2 NaOH H2SO4 + 2 NaOH 3. For breakfast, Robert takes a loaf of bread out of the freezer. 1. 2. 3. 4. He lets the loaf defrost on the counter. He cuts several slices of bread. He toasts the slices in the toaster. He spreads them with butter, which quickly melts on the hot toast. In which step did a chemical change occur? A) 1 B) 2 C) 3 D) 4 4. You find a bottle containing an unidentified liquid. By using universal indicator paper, you determine that the pH of this liquid is 11. Therefore you have to neutralize it before disposing of it. Which of the following methods can be used to neutralize the liquid? A) Add a solution of NaOH. B) Add distilled water. C) Add a solution whose pH is 5. D) Add a solution whose pH is 8. 5. During an experiment, a glass rod, a piece of tin, some copper powder and some rubbing alcohol were each heated separately. The following changes were observed: 1. the glass rod became flexible; 2. the piece of tin turned to liquid; 3. the copper powder turned black; 4. the rubbing alcohol caught on fire. Which of these changes are physical? A) 1 and 2 B) 1 and 3 C) 2 and 4 D) 3 and 4 6. Label Characteristic a. Has 5 protons and 6 neutrons. b. Is an inert gas. c. Has the following electron configuration : 2 6 d. Is an alkaline-earth element. e. Can place its electrons in the first two energy levels. f. Has 2 valence electrons and a complete outermost energy level. g. Is a soft metal, is very reactive and is stored in oil. h. Must lose one electron to obtain the electron stability of element b. i. Is the lightest gas. j. Belongs to the same family as element d and has an atomic number greater than that of element a. k. Is a metalloid. l. Is a halogen. 7. Which of the following substances is a salt? A) KCl B) HNO3 C) LiOH D) SO2 Name of Element 8. The electrolysis of dihydrogen oxide (H2O) is carried out in the laboratory. Dioxygen (O2) and dihydrogen (H2) are obtained. Which of the following diagrams best represents this reaction? ( : oxygen : hydrogen) A) + B) + C) + + D) Energy + Energy + Energy + + Energy 9. The reaction caused by the burning of butane in air is represented by the following equation: 2 C4 H10(g) + 13 O2(g) 8 CO2(g) + 10 H2 O(g) + Energy During a laboratory experiment, you react 29 g of butane (C4H10) in the presence of dioxygen (O2). You observe that 88 g of carbon dioxide (CO2) and 45 g of water vapour (H2O) form. What mass of dioxygen did you use in this experiment? A) 59 g B) 104 g C) 133 g D) 162 g 10. You prepared an aqueous solution of sodium hydroxide, NaOH, that has a concentration of 15 g/L. To do this, you used 60 g of NaOH. What is the volume of the solution you prepared? A) 0.25 L B) 0.90 L C) 1.5 L D) 4.0 L 11. The following table gives the colours of the indicator bromothymol blue in solutions whose pH values vary from 0 to 14. pH 1 Colour 3 5 Yellow 7 9 Green What is the interval of the turning point of bromothymol blue? A) From pH 0 to pH 7.6 B) From pH 6.0 to pH 7.6 C) From pH 6.0 to pH 14.0 D) From pH 7.6 to pH 14.0 12. Draw the lewis dot diagrams for: A) Carbon C) Nitrogen B) Beryllium D) Potassium 11 Blue 13 13. We use a 1000 W microwave oven for 6 minutes, the quantity of energy consumed would be? 14. A nine volt battery supplies power to a cordless curling iron with a resistance of 18 ohms. How much current is flowing through the curling iron? 15. Draw a Series circuit and a parallel circuit, including a power supply. A) Series B)Parallel 16. Change the following concentrations to ppms. A) 10 g/L B) 5 % m/V 17. Determine the charges on the following atoms and give an example of an element that would form an ion with this charge. Example: An atom having lost two electrons ___2+___ ___Mg___ 1. An atom having lost three electrons 2. An atom having gained one electron 3. An atom having gained three electrons _________ _________ _________ ________ ________ ________