CH 17 Thermochemistry
advertisement

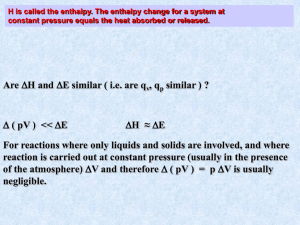
CH 17 Thermochemistry Thermochemistry- energy change that occurs during a chemical reaction and changes of state. - Stored in chemical bonds and is called chemical potential energy Heat- represented by the letter q - Transfer of energy between two objects - Always flows from warmer to colder objects There are two parts to heat: System- what you are focusing on Surroundings – the area around the system All thermal reactions must obey the law of conservation of energy. Therefore, - energy released by the surroundings, must be absorbed by the system (endothermic) - energy released by the system is absorbed by the surroundings. (exothermic) Heat is measured in calories (cal) or joules (j) 1 J = .2390cal and 1 cal = 4.184 J Joules are smaller than calories. Foods calories are kilocalories. Heat capacity and specific heat. Heat capacity- the amount of heat needed to raise the temperature of an object 1 oC. - This is different for all objects and the size is not standardized. We use specific heat instead. Specific Heat is the amount of heat required to raise 1 g of substance 1 oC. Formula : C= q________ M x ∆ T (Change in temp) C = specific heat found on a chart. Q = heat added to system ( J or cal) M =mass in g or kg Change in temp.= ending temp – starting temp Calorimetry is the process by which we measure heat transfer Uses a calorimeter to measure Two types - Constant Pressure- Measure enthalpy (H) of the system. - Equation q = H these are used interchangeably - Q system = H =- q surr = - m x C x T - H is negative fro exothermic reations and positive for endothermic. Constant Volume Calorimeters – - Sample is burned and the surrounding water heats up. - Change in water indicates calories Problems pg 513 Thermochemical equations Heat becomes a reactant or a product in thermochemical reactions. For endothermic reactions, heat is a reactant and H is positive. For exothermic reactions, heat is a product and H is negative. Usually with reactions the change in temp is included beside the completed equation. Look on page 514 and 515. The physical state of the reactants and products must also be listed. Problems on page 516 Heat of combustion Heat required to burn 1 mole of a substance Heat in Changes of State Heat of Fusion and Solidification. The heat needed to melt 1 mole of a substance is the molar heat of fusion (Hfus) The heat needed to freeze 1mole of a substance is the molar heat of solidification( Hsolid) Hfus = -Hsolid The melting of ice to water requires 6.01 kJ/mol The freezing of water to ice requires –6.01 kJ/mol Problems p521 Heat of Vaporization and Condensation Same as the molar heat of fusion and solidification Heat needed to vaporize 1 mole of a substance is the heat of vaporization Heat needed to condense 1 mole of substance is the heat of condensation. For water it is 40.7 kJ/mol Problems pg 524 Heat of solutionThe amount of heat generated by the dissolution of 1 mole of substance NaOH(s) Na+ + OH- H soln = 445.1kJ/mol If the heat of solution is negative the reaction is exothermic If the heat of solution is positive the reaction is endothermic Problems p 526 Calculating Heat of Reactions Hess’s Law of Heat Summation - Two thermochemical equations can be used to determine a final equation, the two heats of reaction can be added to give a final heat of reaction. - Ex C(s) diamonds C(s) graphite o A) C(s) graphite + O2 CO2 H = - 393.5kJ o B) C(s) diamonds + O2 CO2 H- 395.5 kJ o Write equation A in reverse o CO2 C(s) graphite + O2 H = 393.5kJ (kJ is positive because of the reverse reaction) o Add the two equations: CO2 C(s) graphite + O2 H = 393.5kJ + C(s) diamonds + O2 CO2 H- 395.5 kJ C(s) diamonds C(s)graphite H = -1.9kJ Heat of Formation - Hf0= heat to form 1 mole of substances - Hf0 = 0 for elements in their natural state , elements and diatomic elements. The enthalpy, or change in heat can be determined by the following equation. Enthalpy = the heat of formation(Hf0)of the products – heat of formation(Hf0) of the reactants. Problem p. 531 CO (g) + O2 (g) CO2 (g) Balance the equation first. 2CO (g) + O2(g) 2 CO2 (g) Hf0 reactants and products is found on table 17.4 on page 530. For CO it is –110.5 kJ O2 = 0 kJ because it is in it’s natural state. CO2 = -393.5 kJ 2 moles of CO at –110kJ/ mol = -221.0 kJ 2 mole of CO2 at –393.5 kJ/ mol = -787.0 kJ So if we plug in the numbers for the equation: Enthalpy = (Hf0)of the products – (Hf0) of the reactants. Enthalpy = -787.0 kJ-(-221.0 kJ+ 0) = H = -566kJ