CH231-WQ2-2007
advertisement
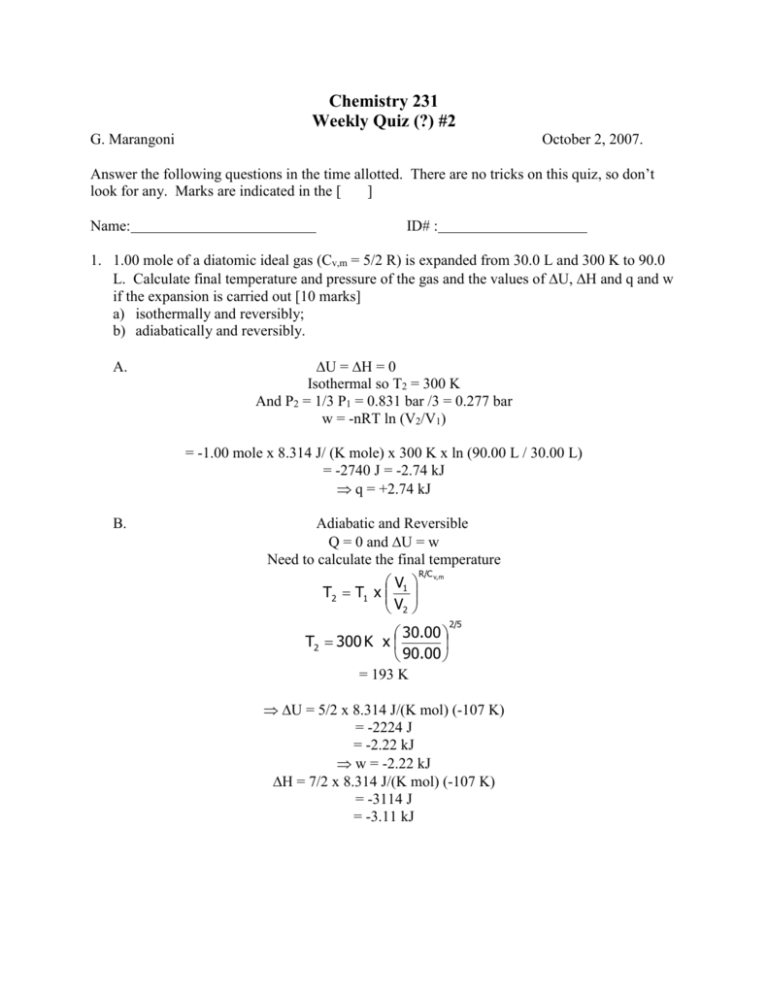
Chemistry 231 Weekly Quiz (?) #2 G. Marangoni October 2, 2007. Answer the following questions in the time allotted. There are no tricks on this quiz, so don’t look for any. Marks are indicated in the [ ] Name: ID# : 1. 1.00 mole of a diatomic ideal gas (Cv,m = 5/2 R) is expanded from 30.0 L and 300 K to 90.0 L. Calculate final temperature and pressure of the gas and the values of U, H and q and w if the expansion is carried out [10 marks] a) isothermally and reversibly; b) adiabatically and reversibly. A. U = H = 0 Isothermal so T2 = 300 K And P2 = 1/3 P1 = 0.831 bar /3 = 0.277 bar w = -nRT ln (V2/V1) = -1.00 mole x 8.314 J/ (K mole) x 300 K x ln (90.00 L / 30.00 L) = -2740 J = -2.74 kJ q = +2.74 kJ B. Adiabatic and Reversible Q = 0 and U = w Need to calculate the final temperature V T2 T1 x 1 V2 R/C v, m 30.00 T2 300 K x 90.00 = 193 K 2/5 U = 5/2 x 8.314 J/(K mol) (-107 K) = -2224 J = -2.22 kJ w = -2.22 kJ H = 7/2 x 8.314 J/(K mol) (-107 K) = -3114 J = -3.11 kJ P2 Lbar x193 K nRT2 1.00 mol x 0.08314 K mol V2 90.00 L 0.178 bar











