Name Chapter 11
advertisement

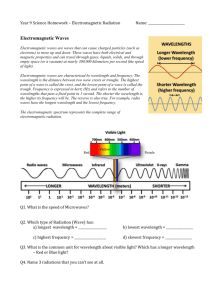
Name _____________________________________ Chapter 11 1. What are four properties of electromagnetic waves? 2. What are the major regions of the electromagnetic spectrum? 3. We cannot see the electrons in an atoms. How can we find how the electrons behave? 4. How have our views on “what light is” changed over the last 200 years? 5. Describe the two components of an electromagnetic wave. 6. Define the following: A) amplitude B) wavelength C) frequency D) Speed of light 7. What property of wave affects the brightness of light? 8. What units are used to measure the frequency of light? 9. What is the speed of light? 10. What color of light has the longest wavelength? 11. What color of light has the shortest wavelength? 12. What device produces infrared radiation? 13. Give a source of ultraviolet radiation? 14. What form of electromagnetic radiation has the shortest wavelength? The longest? 15. When metals are heated, describe what happens as the temperature increases? 16. What happens when metals like barium and strontium are heated? 17. What is a quantum? 18. Who proposed the quantum theory? 19. How is frequency related to the wavelength for a particular radiation? 20. How can astronomers use Planck’s theory while studying electromagnetic radiation emitted by planets and stars? 21. Give an example about something in your daily life being quantized. 22. Why don’t we see the quantum effect in our daily life? 23. For what types of particles is the quantum effect significant? 24. What scientist used the quantum theory to explain the photoelectric effect? 25. What is the photoelectric effect? 26. What name is given to quanta of light? 27. When light shines on sodium metal, will any color of light cause emission of electrons? 28. Explain your answer to the above question? 29. What did Compton believe about light? 30. What does it mean to speak of light having a dual nature? 31. Why does the wavelength of light given off by a hot metal depend on temperature of the metal? 32. Why do strontium and barium give off light when heated? 33. There is an inverse photoelectric effect. Describe how you think it works. 34. What are the three types of spectra? Describe each. 35. Give 3 uses of spectra. 36. Why do atoms give off spectra ( colored lines?) 37. Describe the Rutherford model of the atom. 38. Describe the Bohr model of the atom. 39. What is meant by the ground state of an electron? 40. What is meant by the excited state of an electron? 41. What happens when an excited atom returns to its ground state? 42. Why does hydrogen have three colors of lines and only one electron? 43. Who proposed matter waves? 44. Who proved matter waves? 45. What are matter waves? Why are they not significant for most matter?