Ch5Sec1And2ElectroSpectrum
advertisement

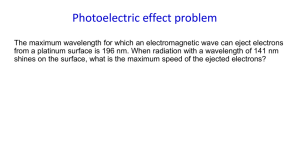
Chapter 5 Section 5.1 Electromagnetic Radiation Section 5.2 Atomic Spectra and Energy Levels Problems with the Rutherford Nuclear Model: Where are the electrons? Why don’t the electrons get pulled into the nucleus? No explanation for spectral lines. *Light behaves as both a wave and a particle. Sometimes light behaves like a wave. Visible light is only a small part of the electromagnetic spectrum. Electromagnetic Spectrum: All the forms of energy that exhibits wavelike behavior as it travels through space. Examples: Gamma rays (short wavelength, high frequency) X-rays Ultra violet Visible (from short to long wavelength - Violet,I,B,G,Y,O,Red) Infrared Microwave TV Radio (long wavelength, low frequency) Speed of all electromagnetic radiation (light): c = 3.0 x 108m/s) Wavelength (λ): The distance between corresponding parts of adjacent waves, e.g. crest to crest. Usually in units of nm Frequency (ν): Number of waves that pass a given point in a given time Usually in waves/second or Hertz (Hz) Speed of Light (c): Speed of light = wavelength x frequency c = λv What is the relationship between the wavelength and frequency? Inverse relationship! - the shorter the wavelength the higher the frequency (as λ ↑ , v ↓) - the longer the wavelength the lower the frequency (as λ ↓, v ↑) What is the frequency of a light wave with a wavelength of 106 nm? Solution: 1. Convert nm to m: 106 nm x (1m/1,000,000,000nm) = 1.06 x 10-7m 2. Solve the light equation for v. c = λv v =c/λ = 3.0 x 108 m/s / 1.06 x 10-7m = 2.83 x 1015 Hz Sometimes light behaves like a particle. Photoelectric effect: the emission of electrons from a metal when light shines on the metal. http://wps.prenhall.com/wps/media/objects /439/449969/Media_Portfolio/Chapter_05/ Photoelectric_Effect.MOV Max Planck Light energy exists in specific small amounts called quanta (quantum – singular) quantum: the minimum quantity of energy that can be lost or gained by an atom. E=hv Planck’s constant, h = 6.626 x 10-34 J•s Determine the energy in joules of a photon whose frequency is 3.55 x 1017 Hz. Solution: E = hv E = (6.626 x 10-34 J•s) (3.55 x 1017 Hz) = 2.35 x 10-16 J Einstein: Won the Nobel Prize for explanation of photoelectric effect. Einstein explained the photoelectric effect by proposing that electromagnetic radiation is absorbed by matter only in whole numbers of photons. Photon: Massless particle of light with a quantum of energy. The energy of the photon depends on the frequency of radiation. Only certain frequencies are energetic enough to knock electrons off the surface of metals. Flame Test: Metal ions produce characteristic colors in a flame. NaCl SrCl2 BaSO4 Line Emission Spectrum: Produced when the light given off by an element is separated into a set of colored lines that are a unique “finger print” of the element. Bohr Model of the Atom Niels Bohr applied quantum theory to the Rutherford model. 1. Electrons exist only in definite orbits. Each orbit has a definite fixed energy. Labeled n = 1,2,3,…etc. Lowest energy level is called the ground state, closest to the nucleus. 2. As long as electrons stay in one energy level they don’t gain or lose energy. Bohr Cont. 3. Adding energy can put atoms in an excited state, the electrons move to a higher energy level, farther from the nucleus. 4. Electrons can “fall” back from higher (excited) to lower energy levels. The difference in energy is released as electromagnetic radiation (light). Explains the color of the lines in the hydrogen spectrum.