CHAPTER 5 READING GUIDE
advertisement

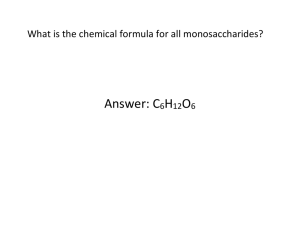
CHAPTER 5 READING GUIDE NAME ______________________________________DATE _________________________ PER _____ POLYMER PRINCIPLES 1. Write a statement that show the relationship between the following: monomers, polymers, and dehydration synthesis. 2. What occurs during a dehydration synthesis reaction? 3. What happens during hydrolysis? CARBOHYDRATES: FUEL AND BUILDING MATERIALS 4. What is the basic monomer of carbohydrates? 5. What is the generalized formula of a monosaccharide? 6. a. What functional groups are found on a monosaccharide? b. Which one determines the type of monosaccharide? 7. What form do sugars have in aqueous solutions? How is this accomplished? 8. Monosaccharides are important forms of cell energy. How do they aid in the formation of other macromolecule? 9. What type of bond connects monosaccharides to form a disaccharide? 10. What monosaccharide bonds form the following disaccharides? Where can they be found? Maltose _______________________________________ __________________________________________________ Sucrose _______________________________________ __________________________________________________ Lactose _______________________________________ __________________________________________________ 11. Plants manufacture food. What is the carbohydrate that they use to transport that food? 12. What are the two roles of polysaccharides? a. 13. ____________________________________________ b. ___________________________________________ Starch is the storage polysaccharide of plants. List the two forms of starch and describe their shape. a. ___________________________ _________________________________________________________ b. ___________________________ _________________________________________________________ 2 14. a. What polysaccharide is used by animals to store excess carbohydrates? b. Where is it stored? 15. What major component of plant cell wall is a structural polysaccharide? 16. See figure 5.7 to answer the following questions. a. What is the main difference between alpha and beta glucose rings in terms of the hydroxyl group? b. Describe how the placement of the glucose monomers in starch and cellulose is different. 17. What causes starch and cellulose to have different distinct three dimensional shapes? 18. A single molecule of cellulose is not significantly strong yet we all recognize the strength of wood. How are cellulose molecules arranged to achieve such great strength? 19. Why can’t we digest cellulose? 20. How can the following animals gain nourishment from cellulose? 21. a. cow b. termite The following questions are about chitin. a. What organisms use this structural polysaccharide? b. How are the glucose monomers of chitin different from the glucose monomers of cellulose? LIPIDS – DIVERSE HYDROPHOBIC MOLECULES 22. What is a defining characteristic of lipids? 23. List the three most important families of lipids. 24. What are the two subunits of a fat? 25. Answer the following questions about fatty acids. a. Why are they called acids? b. Why are they hydrophobic? 26. How is a triacylglycerol made? 27. What is the difference between a saturated fatty acid and an unsaturated fatty acid? 3 28. Why are saturated fatty acids solid at room temperature but unsaturated fatty acids are oils at the same temperature? 29. What does hydrogenated vegetable oil mean? 30. List several important functions of fats in humans. 31. How is a phospholipid different from a fat? 32. Why do phospholipids have ambivalent behavior towards water? 33. What cellular structure has phospholipids as a major component? 34. Why are steroids classified with lipids? 35. What are two important uses of cholesterol? PROTEINS – MANY STRUCTURES, MANY FUNCTIONS 36. 37. List seven functions of proteins. ___________________ _____________________ ______________________ ___________________ _____________________ ______________________ a. What are the monomers of proteins? b. How many are there? __________________ 38. Draw a generalized amino acid and label the amino group, asymmetrical α carbon, carboxyl group, and side chain/ R group. 39. Which unique part of an amino acid determines its chemistry? 40. List the four types of side chains and explain how they will respond to being in an aquatic environment. Side chain type in aqueous environment 41. ____________________________ _____________________________________________________________ ____________________________ _____________________________________________________________ ____________________________ _____________________________________________________________ ____________________________ _____________________________________________________________ How can an amino acid act as a buffer? 4 42. 43. What part of an amino acid will be ionized at the following pHs? Acid _________________________________________________________________ Neutral _________________________________________________________________ Base _________________________________________________________________ a. What is the name of the bond that connects adjacent amino acids during polymerization? b. Explain why proteins are sometimes referred to as polypeptides. 44. What determines the functional three dimensional structure of a protein? 45. Describe the primary structure of a protein. 46. The amino acid sequence of the protein hormone insulin was the first primary structure fully described. a. Who was the researcher? b. How was the sequence determined? 47. Who are involved in creating the secondary structure? What type of bond is created by this relationship? 48. a. Describe the alpha helix. b. List two important structural proteins that have a helical structure in their functional form. _________________________ 49. _________________________ a. Describe a beta pleated sheet. b. List an example of a structural protein with this functional form. 50. What part of an amino acid interacts with other amino acids to form the tertiary structure? 51. Use figure 5.22 to describe interactions that contribute to the tertiary structure of a protein. a. ___________________________________________________________________________________________________ b. ___________________________________________________________________________________________________ c. ___________________________________________________________________________________________________ d. ___________________________________________________________________________________________________ e. ___________________________________________________________________________________________________ 5 52. 53. Disulfide bridges form strong covalent bonds the “rivet” a tertiary structure together. a. What functional group is involved in forming these “bridges”? b. What amino acid has this functional group? a. What is a quaternary structure? b. List two important human proteins that have quaternary functional forms. 54. What is protein denaturation? 55. List three ways to denature a protein. What is the effect of each method on the protein? Method effect ___________________________ _____________________________________________________________________ ___________________________ _____________________________________________________________________ ___________________________ _____________________________________________________________________ 56. How do chaperonins assist in protein folding? 57. What technique is used to determine the shape of a protein? NUCLEIC ACIDS – INFORMATIONAL POLYMERS 58. List the two types of nucleic acid. 59. What is the name of the monomer of nucleic acids? 60. Nucleotides are named by their nitrogen bases. a. What are the three single carbon ring pyrimidines? b. 61. What are the two double carbon ring purines? Pyrimidines only bond with purines. Which pyrimidines will form bonds with a. adenine? ________________________________ b. guanine? ____________________________________ 62. A nucleotide sequence on DNA is T C A T G A C, what is the nucleotide sequence of the complimentary strand? 63. What kind of bond will connect adjacent nucleotides in a nucleic acid? 64. See figure 5.29 to answer the following question. The carbon atoms of the pentose ring of ribose sugar contain significant elements. What is attached or determined at each of the following ribose carbons? C1 _______________________________________________________________________ C2 _______________________________________________________________________ C3 _______________________________________________________________________ C4 _______________________________________________________________________ C5 _______________________________________________________________________