2a_Wet Lab_Determining the Empirical Formula of MgO_Teacher
advertisement
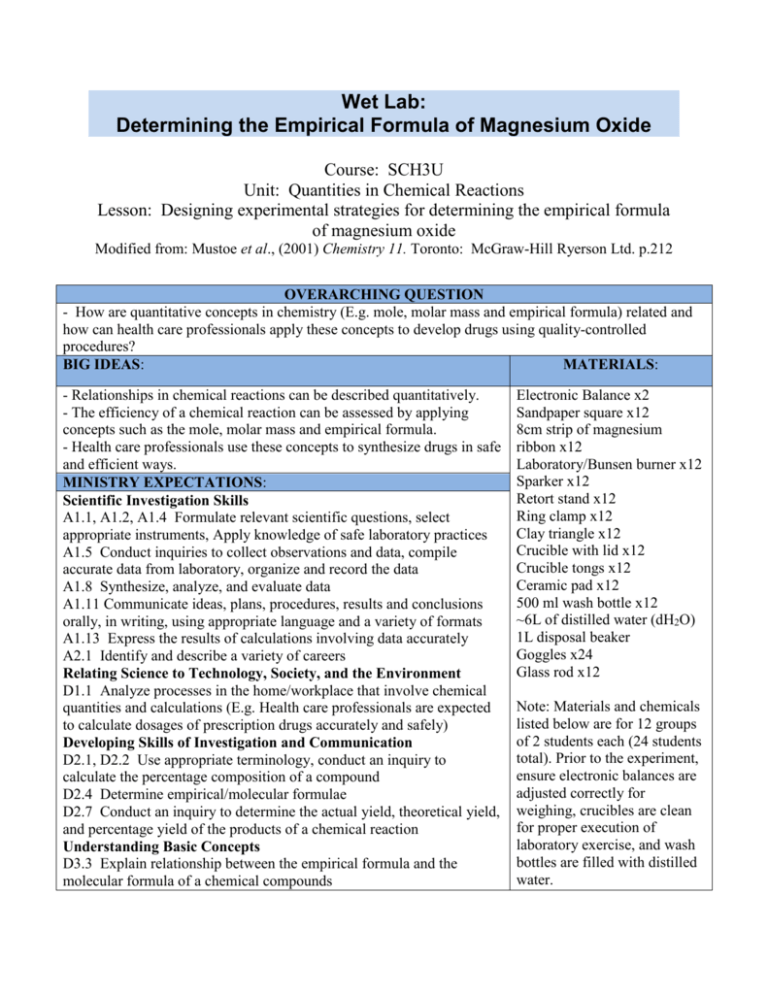
Wet Lab: Determining the Empirical Formula of Magnesium Oxide Course: SCH3U Unit: Quantities in Chemical Reactions Lesson: Designing experimental strategies for determining the empirical formula of magnesium oxide Modified from: Mustoe et al., (2001) Chemistry 11. Toronto: McGraw-Hill Ryerson Ltd. p.212 OVERARCHING QUESTION - How are quantitative concepts in chemistry (E.g. mole, molar mass and empirical formula) related and how can health care professionals apply these concepts to develop drugs using quality-controlled procedures? BIG IDEAS: MATERIALS: - Relationships in chemical reactions can be described quantitatively. - The efficiency of a chemical reaction can be assessed by applying concepts such as the mole, molar mass and empirical formula. - Health care professionals use these concepts to synthesize drugs in safe and efficient ways. MINISTRY EXPECTATIONS: Scientific Investigation Skills A1.1, A1.2, A1.4 Formulate relevant scientific questions, select appropriate instruments, Apply knowledge of safe laboratory practices A1.5 Conduct inquiries to collect observations and data, compile accurate data from laboratory, organize and record the data A1.8 Synthesize, analyze, and evaluate data A1.11 Communicate ideas, plans, procedures, results and conclusions orally, in writing, using appropriate language and a variety of formats A1.13 Express the results of calculations involving data accurately A2.1 Identify and describe a variety of careers Relating Science to Technology, Society, and the Environment D1.1 Analyze processes in the home/workplace that involve chemical quantities and calculations (E.g. Health care professionals are expected to calculate dosages of prescription drugs accurately and safely) Developing Skills of Investigation and Communication D2.1, D2.2 Use appropriate terminology, conduct an inquiry to calculate the percentage composition of a compound D2.4 Determine empirical/molecular formulae D2.7 Conduct an inquiry to determine the actual yield, theoretical yield, and percentage yield of the products of a chemical reaction Understanding Basic Concepts D3.3 Explain relationship between the empirical formula and the molecular formula of a chemical compounds Electronic Balance x2 Sandpaper square x12 8cm strip of magnesium ribbon x12 Laboratory/Bunsen burner x12 Sparker x12 Retort stand x12 Ring clamp x12 Clay triangle x12 Crucible with lid x12 Crucible tongs x12 Ceramic pad x12 500 ml wash bottle x12 ~6L of distilled water (dH2O) 1L disposal beaker Goggles x24 Glass rod x12 Note: Materials and chemicals listed below are for 12 groups of 2 students each (24 students total). Prior to the experiment, ensure electronic balances are adjusted correctly for weighing, crucibles are clean for proper execution of laboratory exercise, and wash bottles are filled with distilled water. STUDENT LEARNING GOALS: APPENDICES 1. Students learn to develop strategies for solving calculation questions Wet Lab – Handout package 2. Students apply calculation strategies properly using correct units and (teacher copy) mathematics Wet Lab - Handout package (student copy) PRIOR KNOWLEDGE: Mole conversions using molar mass and Avogadro’s number Calculations using percent yield Knowledge of experimental apparatus and WHMIS PART T/L STRATEGIES ACTION Apparatus Design (Day 1): - Students review concepts about the production of magnesium oxide and calculating 60 min. empirical formula - Determine apparatus for measuring empirical formula Conduct Experiment (Day2): Students conduct inquiry, and critically analyze results Recommendations (Day3): Students write recommendation letter justifying their claims Inquiry/Research Process and Scientific Method will guide students on how to implement a proper laboratory experiment and evaluate the findings. Students will develop higher-order thinking skills through synthesis of information collected, formulating conclusions, and transfer of prior knowledge and skills to a new situation. They will perform observations, analyze quantitative data, and explain their results using prior knowledge and formulas. This model will aid students in developing and promoting information literacy skills. The Problem-Based Model will help make this laboratory experiment more relevant to students. The lab incorporates a social and cultural concern of high consumption of carbonated drinks (connection to STSE) that can be a cause of stomach ulcers. This model brings together the Ontario curriculum and a real-life issue that will help students extend their knowledge beyond the classroom. Students will determine how stomach ulcers arise and develop a protocol for creating an antacid medication to help solve this issue. Assessment Students will be provided with a handout to be completed prior to the lab. This handout contains scaffolding questions to help students organize the information in a clear, logical manner. It will include questions to assess prior knowledge of chemical reactions and quantities in chemical reactions (AfL). Students will also be responsible in developing a protocol on how to determine the empirical formula of MgxOy(s). To be inclusive of multiple abilities, they will decide how to present the procedure; for example, a visual format (ex. a flow chart and diagram of the apparatus) or a series of written steps. Students will then compare their protocol with the protocol provided by the teacher and make the necessarily adjustments (AaL). This handout will help students self-assess what they already know and what they should improve on to successfully carry out the experiment During the lab, students will be making observations and recording data. They will use this information to answer several questions directly related to the experiment. All components of the experiment (i.e. student handout, data collection, and data analysis) will be collected by the teacher for evaluation (AoL) – see attached rubric for more details. This experiment will allow the teacher to assess students’ knowledge and application of empirical and molecular formulas. Teaching Notes This laboratory experiment fits into the Quantities in Chemical Reactions unit plan after students have a clear understanding of mole conversions, molar mass and empirical formulae concepts. Students continue to build on this existing knowledge in a laboratory setting in order to solidify and contextualize their theoretical knowledge. First, students design and implement a procedure for determining the empirical formula of MgO(s) so that they build additional inquiry skills that allow them to solve empirical formula-based problems through experimentation. They not only have to calculate the empirical formula of a compound quantitatively using mole conversions but they must also interpret their results, suggest sources of error, and reflect scientifically on the strengths and weaknesses of their procedure. Second, students directly link their prior knowledge to practical, real-world issues by applying their skills to antacid production in the pharmaceutical industry. This contextualization helps students make sense of their learning and creates a more engaging classroom environment where students can draw on their own experiences. TEACHER KNOWLEDGE, SKILLS AND PREPARATION NEEDED: Background Knowledge: Combustion is a reaction of oxygen in the air with a sample of matter. In this experiment, the empirical formula of magnesium oxide will be determined. This experiment covers two main areas: i. Law of conservation of mass total mass of the products of the reaction must equal the total mass of the reactants ii. Law of constant composition any potion of a compound will have the same ratio of masses as the elements in the compound The empirical formula of a compound gives the simplest positive integer ratio of atoms of each element found in a compound. The molecular formula, on the other hand, identifies the number of each type of atom found in the molecule. In this laboratory, the magnesium strip is oxidized by oxygen in the air to produce magnesium oxide. Since the air also consists of nitrogen, the magnesium also produces magnesium nitride. Although nitrogen is fond in higher amounts in the air than oxygen, magnesium reacts with less nitrogen than oxygen because oxygen is more reactive. In this experiment there is a purification step to remove any magnesium nitride formed. This is done by washing the product with distilled water and further reheating it. The water converts the magnesium nitride to magnesium hydroxide and ammonia gas. By further heating the product, water is removed and the magnesium hydroxide is converted back to magnesium oxide. The unbalanced equations of the reactions are as follows: Mg (s) + N (g) + O2 (g) MgO (s) + Mg3N2 (s) MgO (s) + Mg3N2 (s) + H2O (l) MgO (s) + Mg(OH)2 (s) + NH3 (g) MgO (s) + Mg(OH)2 (s) MgxOy (s) + H2O (g) Assessment: Teacher should make sure to check and verify student’s protocol for carrying out the lab. Give student the ‘correct’ procedure so that they can compare and correct any mistakes and forgotten steps. Make sure students follow the correct procedure. Safety: Students should be following the following safety precautions at all times during the experiment: - Eye protection at all times – do NOT look directly at the burning magnesium - Open flame so be aware and cautious - Do not breathe in the fumes generated after heating - Do not place anything hot on the balance - Use tongs when handling anything hot such as the crucible, lid, triangle, ring and stand Disposal: All contents are safe to be disposed of into the garbage bins. LABORATORY SET-UP AND CLASSROOM FLOOR PLAN Distribution of materials: The materials required for this lab are located in the labelled cupboards beneath lab bench stations 1 and 2. Bunsen or laboratory burners are located beneath lab bench station 3. All safety equipment (ex. gloves and goggles) is located beneath lab bench station 12. Students are responsible for obtaining and returning these materials to their appropriate place. Sandpaper squares and electronic balances will be supplied by the teacher and will be available on the teacher’s desk. Students are not to remove the balances. A disposal beaker (1L) labelled “Chemical Waste” will be placed in the fume hood. Beakers are located beneath bench station 11. Distribution of chemicals: Magnesium ribbon strips and 500 ml wash bottles with distilled water (dH2O) are to be supplied by the teacher and will be distributed at the teacher’s desk. Student workspace: Each pair of students will be assigned a workspace according to the numbered bench stations (see laboratory floor plan below) Name: _________________________________________________ Date: ______________________________________ SCH3U Chemistry Laboratory: Determining the Empirical Formula of Magnesium Oxide Introduction THE PROBLEM o Research has found that more and more high school students at University of Toronto Schools are consuming highly acidic foods and drinks (E.g. carbonated beverages) that are causing an increased incidence of ulcers. In fact, so many students are developing ulcers that pharmaceutical companies are struggling to keep up with demands for antacid medications. THE SOLUTION o The team: The Grade 11 class from UTS o The motto: We don’t get ulcers. We give them. o The mission: Develop and test a protocol for making high quantities of the antacid, magnesium oxide, or MgO(s). o The task: Imagine that you are a lab technician at the pharmaceutical company, Merck Frosst. Due to high demands, the company has procured large amounts of solid magnesium metal, Mg(s) and needs to convert it to MgO(s) in the most efficient way possible. You are responsible for developing, testing, and analyzing a protocol for making MgO that you can propose to the Merck Frosst CEO, Kenneth Frazier. STAGE 1. PLANNING AND INITIATING o Design a protocol for making MgO(s) from Mg(s) o Determine factors important for creating MgO(s) from Mg(s) o Identify materials/apparatus needed o Identify potential safety hazards o Verify that the product created has the empirical formula, MgO o Note: This portion of the lab must be verified by your teacher BEFORE proceeding to Stage 2: Implementing and Executing STAGE 2. IMPLEMENTING AND EXECUTING o Execute the protocol created in Stage 1 with modifications from the teacher o Follow appropriate safety guidelines STAGE 3. ANALYSIS AND RECOMMENDATIONS o Analyze the data collected to determine if the product created has the empirical formula, MgO o Answer discussion questions o Write a 250-word letter to the president of Merck Frosst SCH3U Quantities in Chemical Reactions (Teacher Copy) 5 Name: _________________________________________________ Date: ______________________________________ STAGE 1: PLANNING AND INITIATION A. What do I know already? 1. What is the PURPOSE of this experiment: The purpose of this experiment is to determine the empirical formula of MgO. 2. The process by which an element combines with oxygen to form an oxide is called [oxidation] . (Hint: See Nelson textbook on p. 200) 3. Fill in the following table to determine the reactants and products of the reaction. REACTANTS What reactant is Merck Frosst SUPPLYING? [Mg(s)] What other reactants are NEEDED to make MgO? Where might you be able to obtain this reactant? PRODUCTS What is/are the desired PRODUCT(s)? [MgO(s)] [O2(g), atmosphere] 4. Using the reactants and products from the above table, write a balanced chemical equation for the desired reaction. [2Mg(s) + O2(g) 2MgO(s)] 5. The oxidation of solid magnesium ribbon occurs very slowly at room temperature – too slow for industrial purposes. How might this reaction be sped up so that it is useful for Merck Frosst? What equipment would be needed? [Add heat = combustion of magnesium = bunsen burner] SCH3U Quantities in Chemical Reactions (Teacher Copy) 6 Name: _________________________________________________ Date: ______________________________________ B. How will I design a protocol for making MgO(s) from Mg(s)? 6. Using the information from Questions 1-5, MAKE A LIST OF MATERIALS that you think you will need to create MgO(s) from Mg(s). Consider chemicals, apparatus, safety equipment, etc. Bunsen Burner Flint Electronic balance Retort stand Ring clamp 8 cm strip of magnesium ribbon Goggles Crucible tongs Ceramic pad Distilled water Wash bottle Clay triangle Crucible with lid Sand paper/emery paper 7. Now it is time to DEVELOP A PROTOCOL. a. OPTION A: Draw and label a diagram to illustrate the apparatus needed for this reaction to proceed. b. OPTION B: Write out a procedure to illustrate the steps needed for this reaction to proceed. Attach this on a separate sheet of paper. 8. CONSIDER SAFETY PRECAUTIONS: What safety precautions and safety equipment will you need when performing this experiment? Equipment: Safety Precautions: Disposal: Safety goggles, lab coat Do not breathe fumes generated Once burner is lit, assume all equipment is hot. Handle equipment with tongs Never place anything hot on a balance Dispose of the MgO in a beaker designated by teacher SCH3U Quantities in Chemical Reactions (Teacher Copy) 7 Name: _________________________________________________ Date: ______________________________________ C. How will I verify that the product has the empirical formula, MgO? It is possible that the protocol designed in Part A will cause Mg and O2 to combine in a ratio that does not give you MgO. For example: Mg(s) + O2(g) MgxOy (s) where x and y are unknown How could you determine the empirical formula of the product? Develop a protocol by considering the following questions: 1. What quantities can you measure for your product MgxOy(s) and your reactant Mg(s)? [The mass of each] 2. Can any of these properties be measured? If so, how? [Yes, by using an electronic balance] 3. If we assume that none of the magnesium is lost (ie. We start and end with the same weight of magnesium) and the weight of our product is higher, what atom is contributing the extra weight? [The oxygen] 4. If we know the mass of the magnesium and the molar mass of the magnesium, what can we calculate? [The number of moles of magnesium in our product] 5. Can you also calculate your answer to 4. but with oxygen? [Yes] 6. Can you compare the quantities of magnesium and oxygen that you would obtain in 4. and 5.? If yes, how does this help you figure out the empirical formula of the product? [Yes. If they are the same then there is a 1:1 ratio of Mg to O in the product and the empirical formula would be Mg(s) + O2(g) MgO (s). However, if you have twice as many moles of O in the product as the Mg then it would be a 1:2 ratio and the empirical formula would be Mg(s) + O2(g) MgO2 (s).] SCH3U Quantities in Chemical Reactions (Teacher Copy) 8 Name: _________________________________________________ Date: ______________________________________ STAGE 2: EXECUTING AND IMPLEMENTING You have now completed Stage 1: Initiation and Planning and have developed a protocol for creating MgO(s) that has been verified by your teacher. D. What steps am I missing in my protocol? Next, compare the protocol that you developed to the protocol listed below. What steps were similar between the two procedures. What steps were different? Use the following table to make assess how complete your protocol is (AaL). Similarities between your procedure and the sample procedure provided Steps missing in your procedure that should be added Steps that should be removed from your procedure - collect apparatus (Bunsen burner, magnesium, flint, tongs, electronic balance) - using the balance, measure and record the mass of the magnesium strip - light the Bunsen burner and burn the magnesium strip - weigh the remaining magnesium oxide - 8cm magnesium strip, crucible with lid, ceramic plate, sand paper, distilled water, clay triangle, glass rod) - using sand paper, clean the magnesium strip to remove any oxides - measure the empty crucible with the lid and record, also measure the crucible with the magnesium strip added and record - burn the magnesium until the magnesium ignites and cover the crucible with the lid as soon as it ignites for one minute (repeat this until the magnesium no longer ignites) - when the magnesium no longer ignites, heat heat the magnesium for another 45mins - use a glass rod to crush up the contents using distilled water, wash off the glass rod and boil until all the water is evaporated - place the magnesium into a beaker and heat - burn the magnesium for 5 minutes SCH3U Quantities in Chemical Reactions (Teacher Copy) 9 Name: _________________________________________________ Date: ______________________________________ Sample Protocol Materials: Bunsen Burner Flint Electronic balance Retort stand Ring clamp 8 cm strip of magnesium ribbon Goggles Crucible tongs Ceramic pad Distilled water Wash bottle Clay triangle Crucible with lid Sand paper/emery paper Safety Precautions: Wear goggles when burning the magnesium; use tongs to remove the hot crucible; do not breathe in the fumes produced; do not place anything hot on the balance Apparatus: Procedure: 1. Assemble the apparatus as shown in the diagram. 2. Obtain a strip of magnesium and clean it using sandpaper or emery paper to remove any coating of oxide on the strip. 3. Using the electronic balance, measure and record the mass of the empty crucible and lid and record the mass in the table below. Then, add the magnesium strip to the crucible and record the mass of the crucible, lid and magnesium strip in the table below. 4. Remove the lid and place the crucible with the magnesium strip on the clay triangle. Light the Bunsen burner and heat the crucible with a strong flame. Using the crucible SCH3U Quantities in Chemical Reactions (Teacher Copy) 10 Name: _________________________________________________ Date: ______________________________________ tongs, hold the lid and as soon as the magnesium ignites (CAUTION), cover the crucible with the lid. Continue to heat for about 1 minute. 5. Carefully remove the lid (CAUTION). Heat the crucible until the magnesium ignites once more and again quickly cover the crucible. Repeat this heating and covering until the magnesium strip no longer ignites. Heat for another 4-5 minutes with the lid off. 6. Using the crucible tongs, place the crucible on a ceramic plate to cool. 7. When the crucible is cool enough to touch, put it on the bench and carefully grind up the product into small particles using the glass rod. Rinse the glass rod with distilled water into the crucible to remove any particles remaining on it. 8. Make sure to add enough distilled water to the crucible to wet all the contents. This white product is magnesium oxide. The yellowish-orange product is magnesium nitride. * 9. Return the crucible to the clay triangle and place the lid slightly ajar. Heat the crucible gently until the water begins to boil and continue to heat until all the water is evaporated and the product is completely dry. Remove the crucible with the tongs and place it on a ceramic pad to cool. 10. Using the tongs, carry the crucible to the electronic balance and measure and record the mass of the crucible, lid and magnesium oxide in the table below. 11. Put the magnesium oxide in the disposal beaker provided by your teacher. DO NOT put it in the garbage or sink. Note: When weighing out the magnesium ribbon, make sure the mass is at least 0.10g SCH3U Quantities in Chemical Reactions (Teacher Copy) 11 Name: _________________________________________________ Date: ______________________________________ E. What do I observe when I carry out the experiment? Use the following observation table to tabulate the results of your experiment. Mass of clean, empty crucible and lid Mass of crucible, lid and magnesium strip Mass of crucible, lid and magnesium oxide 13.8g 14.0g 14.1g F. How should my data be analyzed? ANALYSIS QUESTIONS: 1. a) Using the mass of magnesium strip used and magnesium oxide produced, calculate the mass of oxygen that reacted with the magnesium. Mg (s) +O2 (g) MgO (s) (0.2g) + (0.1g) (0.3g) Mas of Oxygen that reacted with magnesium = (Mass of magnesium oxide produced, crucible and lid – mass of magnesium, crucible with lid) = 14.1g – 14.0g = 0.1g b) Using this experimental data, calculate the percentage composition of the magnesium oxide. Experimental percentage composition of Mg = (mass of Mg used/mass of Mg0 produced) x 100% = (0.2g/0.3g) x 100% = 66.6% = 67% Experimental percentage composition of O2 = (mass of Mg used/mass of Mg0 produced) x 100% = (0.1g/0.3g) x 100% = 33.3% = 33% SCH3U Quantities in Chemical Reactions (Teacher Copy) 12 Name: _________________________________________________ Date: ______________________________________ c) Determine the experimental empirical formula of magnesium oxide (assume a 100g of MgO). Note: round your empirical formula to the nearest whole number ratio e.g. 1:1, 1:2, 2:1 or 3:3 Ratio of Magnesium: Mass of Mg = 67g Molar mass of Mg = 24 g/mol Number of moles = mass/molar mass = 67g/(24g/mol) = 2.791 mol Ratio of O2: Mass of O2 = 33g Molar mass of O2 = 16g/mol Number of moles = 33g/(16g/mol) = 2.062 mol Determining ratio: O2 = (2.062mol)/(2.062mol) = 1 Mg = (2.791mol)/(2.062mol) = 1.35 = 1 Therefore the ratio is 1:1 and the empirical formula is MgO 2. a) Before moving on, verify your empirical formula with your teacher and use the empirical formula of magnesium oxide to determine the theoretical mass percent of magnesium in magnesium oxide. Theoretical molar mass of MgO = 40g/mol Molar mass of Mg = 24g/mol Percentage composition = (molar mass of Mg/molar mass of MgO) x 100% = [(24g/mol)/40g/mol] x 100% = 60% Mg b) Determine your percent yield by calculating the difference between your actual mass percent of magnesium (AP) and your experimental mass percent of magnesium (EP) using the following formula: Percent error = (EP-AP) x 100% AP = [(67%-60%)/60%] x 100 = 11.7% SCH3U Quantities in Chemical Reactions (Teacher Copy) 13 Name: _________________________________________________ Date: ______________________________________ 3. a) Why did we put water in the crucible and then evaporate it completely? What purpose does this serve? (Hint: Think of what other byproducts were in there) Since the air is composed of not only Oxygen and there is a large component of Nitrogen, the nitrogen also reacts with the magnesium to form Mg3N2 (magnesium nitride). Adding water to the crucible serves as a purification process because the nitrogen reacts with the Hydrogen in the water to produce ammonia gas and the remaining magnesium then reacts with more oxygen b) Why was magnesium nitride (Mg3N2) produced as well as magnesium oxide (MgO)? Because the air is comprised of not only oxygen but also a large component of it is nitrogen as well so some of the nitrogen also reacts with the magnesium to produce magnesium nitride c) Would you expect more magnesium nitride (Mg3N2) to be produced or more magnesium oxide (MgO) to be produced? Why? Although there is more nitrogen present in the atmosphere, more magnesium oxide with in fact be produced because oxygen is more reactive than nitrogen. 4. a) What are some sources of error in this experiment? (list at least 2) 1) Not all the magnesium was burned. 2) Some magnesium oxide smoke escaped during the reaction. b) How could these sources of error be reduced/how did we try to reduce these sources of error? 1) By burning it multiple times until didn’t any more. 2) Putting the lid on as quickly we could when the reaction began. SCH3U Quantities in Chemical Reactions (Teacher Copy) 14 Name: _________________________________________________ Date: ______________________________________ G. What recommendation should I make? TASK: Write a 250-word letter (maximum) to the president of Merck Frosst outlining the need for antacids and the solution that you developed. Make a recommendation on whether or not you think your solution will be effective and support your recommendation with evidence. Be sure to include a short description/explanation of the following: - What antacids are used for? (Why do we need them?) - What you did to produce an antacid? - What the antacid you produced is? - Do you think this will be effective? (Why or why not?) - Give at least one piece of evidence to support your claim Dear Merck Frosst, It has come to my attention that, due to the rising number of stomach ulcers arising from acid reflux, we are in dire need of more antacid medicine that can be made relatively inexpensively and in large quantities. I’m sure you know that magnesium oxide acts as an antacid and is therefore able to fill this need. Thus, my class and I have developed a procedure for manufacturing magnesium oxide (MgO) by only using magnesium (Mg), heat, air, and water. We have ensured that only MgO is produced and that any impurities (such as magnesium nitride) have been removed through a purification step with water. There are many pros and cons to using this to produce antacids. The first is that it is relatively inexpensive as the only material you need to purchase is magnesium. Moreover, if one can obtain a large quantity of magnesium and equipment it is possible to produce this in large quantities. However, the only drawback in using magnesium oxide as an antacid is that it is not supposed to be used repetitively and can be used as a laxative as well (http://www.nlm.nih.gov/medlineplus/druginfo/meds/a601074.html). That being said, it is still used as a viable antacid thus, I believe this is an effective way to fill the need for antacids to battle stomach ulcers. Sincerely, Student PS See enclosed for procedure outline H. References Mustoe, F., Jansen, M. P., Doram, T., Ivanco, J., Clancy, C., & Ghazariansteja, A. (2001). Chemistry 11. (pp. 212-213). Toronto,ON: McGraw-Hill Ryerson Limited. SCH3U Quantities in Chemical Reactions (Teacher Copy) 15 Name: _________________________________________________ Date: ______________________________________ I. Rubric (Pre-lab, Protocol Design, Recommendation Letter) Categories 50-59% (Level 1) Knowledge and Understanding Knowledge of The student content demonstrates limited knowledge about empirical formulas, balancing equations and is unable to answer questions about determining empirical formulas Understanding of content 60-69% (Level 2) 70-79% (Level 3) 80-100% (Level 4) The student demonstrates some knowledge about empirical formulas, balancing equations and is able to answer some questions about determining empirical formulas The student demonstrates considerable knowledge about empirical formulas, balancing equations and is able to answer most questions about determining empirical formulas correctly The student demonstrates considerable understanding about determining empirical formulas The student demonstrates a high degree of knowledge about empirical formulas, balancing equations and is able to answer almost all questions about determining empirical formulas The student demonstrates a high degree of understanding about determining empirical formulas The student uses planning skills with considerable effectiveness and the information is organized in an orderly way The student uses processing skills with considerable effectiveness and shows creativity and detail to the protocol design The student used critical/creative thinking processes with considerable effectiveness in the protocol design The student uses planning skills with a high degree of effectiveness and the information is very organized and easy to follow The student uses processing skills with a high degree of effectiveness and is very creative with a lot of detail in the protocol design The student used critical/creative thinking processes with a high degree of effectiveness in the protocol design The student expresses and organizes ideas and understandings with considerable effectiveness and the recommendation letter contains all required sections but the student does not answer all required questions effectively Expresses and organizes ideas and understandings with a high degree of effectiveness and the recommendation letter contains all required sections and address all required questions effectively The student demonstrates limited understanding about determining empirical formulas The student demonstrates some understanding about determining empirical formulas The student uses planning skills with limited effectiveness and the information is not organized in an orderly way The student uses planning skills with some effectiveness and the information is organized but it fairly disorganized Use of processing skills The student uses processing skills with limited effectiveness and shows little or no creativity and detail to the protocol design The student uses processing skills with some effectiveness and shows some creativity and detail to the protocol design Use of critical/creative thinking processes The student used critical/creative thinking processes with limited effectiveness in the protocol design The student used critical/creative thinking processes with some effectiveness in the protocol design Thinking Use of planning skills Communication Expression The student expresses and and organizes ideas organization of and understandings ideas with limited effectiveness and the recommendation letter does not contain all required sections and does not address all required questions The student expresses and organizes ideas and understandings with some effectiveness and the recommendation letter contains most of the required sections but does not address all required questions SCH3U Quantities in Chemical Reactions (Teacher Copy) 16 Name: _________________________________________________ Communicatio n for different audiences and purposes Date: ______________________________________ The student communicates for different audiences and purposes with limited effectiveness and writes the recommendation letter in an inappropriate tone The student communicates for different audiences and purposes with some effectiveness and writes the recommendation letter in a somewhat appropriate tone The student communicates for different audiences and purposes with considerable effectiveness and writes the recommendation letter in an appropriate tone The student uses conventions, vocabulary, and scientific terminology with limited effectiveness throughout the experiment The student uses conventions, vocabulary, and scientific terminology with some effectiveness throughout the experiment The student uses conventions, vocabulary, and scientific terminology with considerable effectiveness throughout the experiment The student applies knowledge and skills in familiar contexts with limited effectiveness and was unable to design a correct protocol The student applies knowledge and skills in familiar contexts with some effectiveness and was able to design some correct steps for the protocol The student applies knowledge and skills in familiar contexts with considerable effectiveness and was able to design a correct protocol Transfer of knowledge and skills The student transfers knowledge and skills to new contexts with limited effectiveness and shows no signs of applying knew knowledge to design a protocol The student transfers knowledge and skills to new contexts with some effectiveness and shows some signs of applying knew knowledge to design a protocol Making connections within and between various contexts The student makes connections within and between the results obtained in the lab and theory learned in class with limited effectiveness The student makes connections within and between the results obtained in the lab and theory learned in class with some effectiveness The student transfers knowledge and skills to new contexts with considerable effectiveness and shows signs of applying knew knowledge to design a protocol The student makes connections within and between the results obtained in the lab and theory learned in class with considerable effectiveness Use of conventions Application Application of knowledge and skills SCH3U Quantities in Chemical Reactions (Teacher Copy) The student communicates for different audiences and purposes with a high degree of effectiveness and writes the recommendation letter in a very appropriate tone The student uses conventions, vocabulary, and scientific terminology with a high degree effectiveness throughout the experiment The student applies knowledge and skills in familiar contexts with a high degree of effectiveness and designed a protocol that was very accurate correct The student transfers knowledge and skills to new contexts with a high degree of effectiveness and is very effective in applying knew knowledge to design a protocol The student makes connections within and between the results obtained in the lab and theory learned in class with a high degree of effectiveness 17








