Lecture 16 - Pilkington Group
advertisement

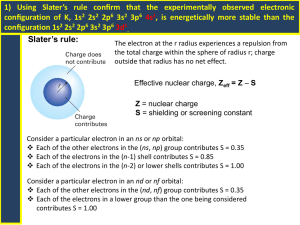
2P32 – Principles of Inorganic Chemistry Dr. M. Pilkington Lecture 16 – “The Network” 1. The periodic table - a group of interconnected organizing ideas that help make sense of inorganic chemical behavior. 2. Effective nuclear charge. 3. Slater’s rule for calculating effective nuclear charge (ENC). 4. Periodic behavior - ionization energy and atomic radii 1. The periodic table Remember that part of the objectives of this course are to develop an interconnected network of ideas that we can use to rationalize and predict a variety of chemical behavior. Descriptive inorganic chemistry h – a network k is essentiall here h to understand and predict the properties, structures, and applications of the elements and their most important properties. In the last half of the 19th century, the development of the periodic table coincided with the emergence of the field of inorganic chemistry. The periodic table- Mendeleev’s masterpiece was the foundation for the organization of any study of the elements and their compounds. 1 To-date the known chemistry of 114 elements is still incredibly rich and diverse and still growing. Where to do we start? – We will begin to build a network of specific organizing ideas on which to base our understanding of the periodic table and the chemistry of the elements. elements To-date we take for granted the arrangement of elements into periods and groups. First, we will review what characteristics and properties of the elements show periodic trends. We begin with with a review of ionization energies, g atomic radii, electron affinities and electronegativities. A keystone in the understanding of periodic properties is the concept of effective nuclear charge. (Zeff) 2. Effective Nuclear Charge (ENC; Zeff) Apparent nuclear charge acting on a given electron is the actual nuclear charge (Z; atomic #) minus a screening constant () that takes into account the effect of the shielding electrons: electrons closer to the nucleus than the electron being considered. Zeff = Z- outer electron does NOT feel the full effect of the protons because of the shielding electron Z+ shielding electron 2 Two methods to calculate the screening constant i. Simple method (used in the first year) Take = an integer that is equal to the number of electrons in the shells below the one being considered. For example: A 18Ar = 1s 1 2 2s 2 2 2p 2 6 3s 3 2 3p 3 6 2nd shell 3rd shell Zeff for a 3s or 3p electron: = 10 (1s22s22p6) i.e. 10 electrons are shielding the outer orbital electrons Zeff = Z - Zeff = 18-10 Zeff = 8 The outer electrons feel a + 8 charge which is the same as the group number.of the element. Zeff for the 2s or 2p electron In this case the only shell below is the 1st shell. Zeff = Z- = 2 (1s2) = 18 -2 = 16 (i.e. it will be harder to remove) ii. More accurate method – use Slater’s rules The rules were devised semi-empirically by John C. Slater in 1930. This takes into account the electrons in the same shell can do some shielding and partial shielding can occur in lower shells. It gives a more accurate representation of the shielding effect on the atoms. atoms When Zeff is large then it is harder to remove the electron. When Zeff is small it is easier to remove the electron. 3 3. Slater’s rules. This set of simple rules for approximating the effective nuclear charge was proposed a number of years ago by Professor John C. Slater, a former faculty member at M.I.T. 1) Write the electron configuration for the atom using the following design; (1s)(2s,2p)(3s,3p) (3d) (4s,4p) (4d) (4f) (5s,5p) 2) Any electrons to the right of the electron of interest contributes no shielding. (Approximately correct statement.) 3) All other electrons in the same group as the electron of interest shield to an extent of 0.35 nuclear charge units 4) If the electron of interest is an s or p electron: All electrons in the next inner most group shield to an extent of 0.85 units of nuclear charge. A contribution of 1.00 units for each electron in still lower or farther in groups. 5) If the h electron l of f iinterest iis an d or f electron: l All electrons l to the h left l f shield hi ld to an extent of 1.00 units of nuclear charge. 6) Sum the shielding amounts from steps 2 through 5 and subtract from the nuclear charge value to obtain the effective nuclear charge. Examples 1. Calculate Zeff for a valence electron in fluorine. (1s2)(2s2,2p5) R l 2d Rule does nott apply; l 0 0.35 35 · 6 + 0 0.85 85 · 2 = 3 3.8 8 Zeff = 9 – 3.8 = 5.2 for a valence electron. 2. Calculate Zeff for a 6s electron in Platinum. (1s2)(2s2,2p6)(3s2,3p6) (3d10) (4s2,4p6) (4d10) (4f14) (5s2,5p6) (5d8) (6s2) Rule 2 does not apply; 0.35 · 1 + 0.85 · 16 + 60 · 1.00 = 73.95 Zeff = 78 – 73.95 = 4.15 for a valence electron. 4 3. Calculate the Zeff for a 4s electron versus a 3d electron in Zn. Cu = [1s]2 [2s,2p]8 [3s,3p]8, [3d]10,[4s]2 Zeff for a 4s electron = 30 – [(1 x 0.35) + (18 x 0.85) + (10 x 1.00)] = 30 – (25.65) = 4.35 Zeff for a 3d electron = 30 – [(9 x 0.35) + (18 x 1)] = 30 – 21.15 = 30 – 21.15 = 8.85 Conclusions the electron that is lost during the ionization is the one that feels the least attraction to the nucleus. 4s electron in Zn feels a charge of + 4.35 3d electron in Cu feels a charge of + 8.85 Generally for transition metals, the 4s electrons are removed before the 3d. 4. i. Periodic Behavior Ionization energy (I.E) 1st I.E = H for the process (enthalpy or energy released) M (g) M(g)+ + e- (g) the removal of the first electron to make a positive ion. (cation energy). 2nd I.E M+ (g) M(g)2+ + e- (g) the removal of the second electron to make a more positive ion. (dication energy). Rodgers, Figure 9.6 pg 225 - plot of 1st ionization energy versus atomic number 5 The plot of the first ionization energies versus atomic number shows some interesting periodic trends that provide information about the structure of atoms. Clearly, the most striking feature of this graph is that there is an inherent periodicity. How can this periodicity be explained? If we examine the electronic configurations of these elements we find that all elements with the lowest ionization energies -the alkali metals - have similar electronic configurations - [noble gas]ns2. Similarly, all the elements with the largest ionization energies - the noble gasesh have filled fill d s and d p electronic l t i configurations fi ti - [noble [ bl gas](nd ]( d10)ns2np ) 2 6. 6 All the highest peaks are at the noble gases – they have the highest Zeff so it is the harder to remove an electron. For the alkali metals Zeff of K is lower than Li and Na due to more shielding. The outer electron is thus easier to remove making K more reactive. The reactivity of the alkali metals can be understood by considering their electronic configurations. First, let's examine the reactivity of lithium, sodium and potassium with water. http://www.iun.edu/~cpanhd/C101webnotes/modern-atomictheory/alkali-reac.html 7 The Zeff of the transition metals vary slowly because the electrons coming in are filling the 3d orbitals. The lanthanides (57-71) the Zeff is almost constant because the added electrons are filling the 4f orbitals which are two levels down from the valence 6s electrons. Question - why are there irregular breaks in the 1st ionization energy of the second period? Consider the electronic configuration of the elements. Be B C N O 2s2 2p1 2p2 2p3 2p4 8 Be: 1s2 2s2 -e- B: 1s2 2s2 2p1 1s2 2s1 -e- 1s2 2s2 Two factors to consider: i. There is a lot of shielding from the inner electrons (compared to Be), away ii. The 2p orbital is also more diffuse so the electron is further away from the from the h nucleus l (inner ( core)) hence h the h outer electron l is more easily l removed. nucleus on average. 2s 2p node - electron density at the nucleus is zero the electron can get closer to the nucleus in the 2s rather than the 2p orbital because of the circular shape. 9 N: [He] 2s2 2p3 O: [He] 2s2 2p4 -e-e- N [He] 2s2 2p2 [He] 2s2 2p3 O 2p 2s 2p 2s In the case of N there are no paired electrons so any of the 2p electrons can be removed because they have the same energy. In the case of O, it is takes less energy to remove the paired electrons since they repel one another, hence the electronic replusion meanse that it requires less energy than would be expected to renive the electron. The pair of electrons in the orbital does have an effect on the IEand ENC. N.B. Slater’s rules for N and O do not take the period paired electrons into account. The σ (and Zeff) are the same. ii. Atomic Radii Ionic radii increase down a group. Ionic radii decrease across a period. Cations are smaller than their parent atoms. Group Period 10 Two factors must be taken into consideration in explaining this periodic trend (1) Increasing nuclear charge (2) Increasing shell Along a period (left to right) the atomic number increases while the valence electrons remain in the same shell. Thus due to the increasing nuclear charge ( lli electrons (pulling l t closer l tto th the nucleus) l ) th the radii dii of f the th atoms t decrease d left l ft to t right. Hence Li = largest in 2nd period. Ne = smallest in 2nd period. Li Zeff = 1.3 Ne Zeff = 5.85 (Ne attracts the electrons more tightly) Top to bottom along a group the atomic number continues to increase. However th shell the h ll increases i from f shell h ll 1 tto shell h ll 2 etc.. t The Th atomic t i orbitals bit l for f each h successive shell get larger and larger - more than compensating for the increased nuclear charge. The result is atomic radii increase top to bottom along a group. Na and Li have the same ENC, so why y is Na is the larger g atom ? 2 2 6 1 Na = 1s 2s 2p 3s Li = 1s2 2s1 This is because the valence electrons of Na is in an orbital with a higher quantum number, i.e. in a 3s orbital that is larger than a 2s orbital. 11