Introduction to Organic Chemistry
advertisement

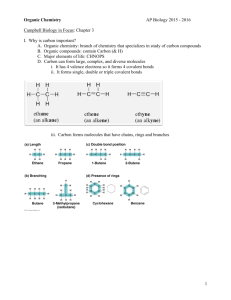
AP Biology I Pennsbury High School Water & Carbon Unit Part 1 2009-10 School Year Introduction to Organic Chemistry What are the factors that contribute to carbon’s unique ability among the elements to produce such a staggering variety of chemical compounds? Organic compounds are compounds of carbon. The organic molecules of living things may contain C, O, H, N, S, and P among other atoms. Even though the molecules of all living things are made up of these same six elements, the atoms of organic molecules can be arranged so many different ways that the uniqueness of each organism is ensured. Carbon’s versatility allows a limited assortment of atomic building blocks, taken in roughly the same proportions, to be used to build an inexhaustible variety of organic molecules. 1. Carbon atoms are tetravalent. That is, they form four bonds. “Intersection point” Valences of atoms provide a set of simple rules that govern how atoms combine to make molecules. 2. Stability of C-C bonds and the ability to form long stable chains. Organic molecules can vary in the length of the carbon chain that makes up the molecule. (See list of word roots for naming alkanes, alkenes, and alkynes of different lengths.) 3. Carbon chains do not have to be straight, they can be branched. Structural isomers. 4. In addition to forming single bonds between carbon atoms, atoms of carbon can also form double and triple bonds with other carbon atoms. 5. When a carbon-carbon double bond exists within a carbon chain, there are two isomers formed resulting from possible variation in the arrangement of chemical groups around the double bond. Geometric isomers (cis-trans isomers). AP Biology I Pennsbury High School Water & Carbon Unit Part 1 2009-10 School Year 6. The position of a carbon-carbon double or triple bond within a chain can vary resulting in structural isomers. 7. When a carbon compound has an asymmetric (chiral) carbon, there will be enantiomers (optical isomers). Enantiomers are distinguished as R (rectus) or S (sinister) isomers. The R and S isomers of a given compound have different biological activities. Enantiomers are mirror images of each other. 8. Different organic compounds can be made by adding different functional groups to the carbon skeletons. Functional groups important in biological molecules include: (hydroxyl, [-OH]; carboxyl, [-COOH]; carbonyl, [-CHO]; amino, [-NH2]; sulfhydryl, [-SH]; phosphate, [-PO43-]; and ester [-R-COO]. 9. In large organic compounds, different compounds are formed when functional groups are located on different carbons on the carbon skeleton. Structural isomers. 10. Carbon skeletons can also exist in ring forms, where one end of the chain of carbons is attached to the other end forming a cyclic compound. E.g., cyclohexane. In addition, there is also an important group of chemical compounds based on a ring compound known as benzene. These are aromatic compounds. AP Biology I Pennsbury High School Water & Carbon Unit Part 1 2009-10 School Year Some concept maps that illustrate the relationships between concepts. Organic Compounds Can be classified as Aliphatic Compounds Aromatic Compounds May be Straight-Chain Cyclic Includes Alkanes Alkenes Alkynes Isomers Can Be Structural Isomers Stereoisomers (Connectivity Differences) (Configuration Differences) Include Branched Chains Include Position of Func. Group Position of Double/Triple Bond Enantiomers Diastereomers (mirror image) (non-mirror image) AP Biology I Pennsbury High School Water & Carbon Unit Part 1 2009-10 School Year Word Roots for Naming Carbon Chains Root meth- Number of Carbons Example of an Alkene methane N/A ethane ethene propane propene butane 1-butene pentane 2-pentene hexane 2-hexene heptane 1-heptene 1 eth- 2 prop- 3 but- 4 pent- 5 hex- 6 hept- Example of an Alkane 7 AP Biology I Pennsbury High School Water & Carbon Unit Part 1 2009-10 School Year Word Roots for Naming Carbon Chains (cont.) Root Number of Carbons oct- 8 non- 9 dec- 10 Example of an Alkane Example of an Alkene octane 4-octene nonane 2-nonene decane 1-decene