Baking Soda - Stoichiometry Lab name: Measure on the scale Mass

F
G
H
D
E
B
C
Baking Soda - Stoichiometry Lab name: _______________________________
During the decomposition of sodium hydrogen carbonate we produced carbon dioxide gas. The other products are murky for you so far. But, given the choice of three possible reactions, and some good measuring on your part, this should be easy.
Baking soda is used in foods because as you heat it up, the decomposition produces the gas, which makes your baked good fluffy (the dough rises).
Baking soda is also known by its old fashioned name: sodium bicarbonate, but we will not call it that in our class, that naming protocol has been abandoned (but not by food chemists).
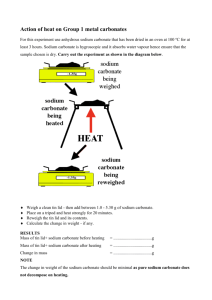
Procedure: Put on your goggles first. Then, set up a ring stand with clay triangle, get Bunsen burner at the ready. Get a crucible with a lid, make sure it’s clean. Get mass of crucible (no lid) when clean and dry.
Measure out between 3.40 grams of sodium hydrogen carbonate (measure to the 100th gram) and make sure it’s ALL inside the crucible and not on the scale.
We will put a lid on the crucible to make sure nothing pops out of the crucible. Gases will escape easily, but any solids that are bounced will bounce back into the cup where we can measure them.
Heat the crucible with lid for three minutes (not crazy hot, just enough to break out the gas. Cool, and then mass with out the lid. Heat again for one minute, cool, mass again without the lid.
Repeat 4 times total. At some point the heating will have caused all the gases to escape, we are seeking that final, unchanging mass. When the mass stops changing (this make take a fifth heating) it’s time to clean up: warm water, soap, put next to sink UPSIDE down to dry. Put equipment away. The “cooked” baking soda can go into the sink or into the trash, it’s not harmful.
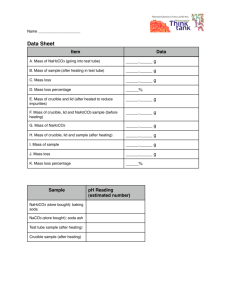
Measure on the scale Mass in grams, 100th gram
A Empty crucible
Crucible and Sodium hydrogen carbonate
Sodium hydrogen carbonate alone (B
A"er first hea$ng, crucible and solid
Mass of le" over solid only (G
-
A)
-
A)
A"er second hea$ng, crucible and solid
A"er third hea$ng, crucible and solid (if necessary)
A"er fourth hea$ng, crucible and solid (if necessary)
3.40 grams
The reason that this is such a cool lab is that you probably don’t know what really happened in that crucible.
Here are three possible reactions, one of them is true.
1. Baking soda decomposes into sodium hydroxide solid + carbon dioxide gas
2. Baking soda decomposes into sodium oxide solid + carbon dioxide gas + water gas
3. Baking soda decomposes into sodium carbonate solid + carbon dioxide gas + water gas
Lab Questions, to be done on loose leaf paper, in order, neatly, with plenty of space to write you back some fun notes and hints on how to think more.
1. Write the balanced chemical equation for possible reaction #1 above.
2. If you used 3.40 g of sodium hydrogen carbonate, how many grams of sodium hydroxide should form?
3. Write the balanced chemical equation for possible reaction #2 above.
4. If you used 3.40 g of sodium hydrogen carbonate, how many grams of sodium oxide should form?
5. Write the balanced chemical equation for possible reaction #3 above.
6. If you used 3.40 g of sodium hydrogen carbonate, how many grams of sodium carbonate should form?
7. Based upon the stoichiometry, and your results from the data table, which of these 3 reactions occurred?
Try to support your choice with a few well worded sentences so I can see if you guessed or if you’re getting it.
8. Calculate your percent error (of grams of solid product forming). %Error gets a sign and proper SF!
9. Why is the mass of the solid product less than the mass of the solid reactant in this decomposition
reaction? Is that okay or does it somehow break the Law of Conservation of Matter?
10. Why is baking soda a common ingredient in cake recipes?
Points for this lab:
Cover with title, nice optional diagram/picture, and mandatory descriptive sentence. = 2 points
Ten questions above x 2 points each = 20 points, and a conclusion for 3 points.
Your Conclusion MUST INCLUDE:
1. Explain the concept of stoichiometry, what is it used for. How did you use it in this lab?
2. Name the 5 kinds of chemical reactions you know already.
3. Explain what the letters of TOPIC-B stand for.
4. Write out the Law of Conservation of Matter. (in full sentences)
5. Write out the Law of Conservation of Energy. (in full sentences)
6. Name 6 kinds of phase changes (physical changes, not chemical changes) in their proper pairs.