Decomposition of Baking Soda Lab
advertisement

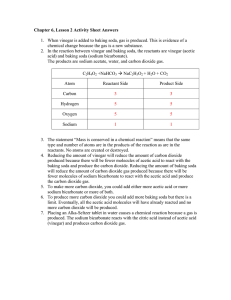
TITLE: DECOMPOSITION OF BAKING SODA (Lab guidelines: sections 1,2,3,5,6,7,9,10,11,12) BACKGROUND: The thermal decomposition of baking soda has been studied extensively by food chemists, due to the widespread use of baking soda in so many food products. Do you know why baking soda is so popular in many baked products? As the temperature of baked items such as cakes increases to 50 C, the baking soda decomposes and releases carbon dioxide. What is the result of carbon dioxide being released? Your mission is to determine what the other products of this reaction are. You will do this using your knowledge of chemical equations and moles. PURPOSE: Use your knowledge of chemical equations and moles to determine which of the following three reactions is correct for the decomposition of baking soda. sodium hydrogen carbonate sodium hydroxide + carbon dioxide sodium hydrogen carbonate sodium oxide + carbon dioxide + water sodium hydrogen carbonate sodium carbonate + carbon dioxide + water NOTE: Before you will be allowed to begin the lab you must write balanced equations for each of the reactions above. Include the word equations and the balanced equations in your purpose section. MATERIALS: 2-3 grams sodium hydrogen carbonate, crucibles (no lid), balance, spatula, ring clamp, ring stand, clay triangle, Bunsen burner PROCEDURE: You must write your own procedure. Please keep the following in mind. What masses will you need to record? You will need to heat your baking soda for 7-10 minutes to ensure the reaction is complete. Allow crucible to cool for several minutes before massing it again. You must underline each of the materials listed above as you write your procedure. During heating, with your lab partner, perform the calculations necessary in order to accomplish the purpose. This is one of the few labs that allows you to do the calculations before the lab is finished -- so take advantage of that!!! DATA: Create a data table and record appropriate data. CALCULATIONS: Perform necessary calculations. Organize and label your calculations. Include units on all numbers!! Show how you know which chemical equation is correct. QUESTION: (actually just more calculations). Use stoichiometry to determine the mass of the other product(s). ERROR ANALYSIS: Calculate your percent error for the process using your data and your calculations. The accepted value for the process is your stoichiometric calculation. Discuss sources of error and their impact. CONCLUSION: Write a conclusion which demonstrates that you understand what you did. How did you accomplish the purposes? Discuss the math as well. Remember, here is a great guide for writing your conclusion: 1) Restate the purpose 2) Summarize the procedure and data collection 3) Discuss any important principles / equations used 4) Explain the graphs and/or math 5) Report your results Also, please include any other interesting information you would like to add.