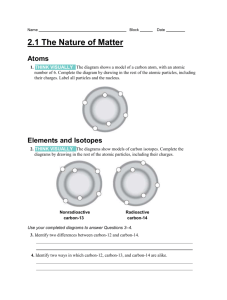
Name Term 2 Practice Final When a sodium atom becomes a

Name _______________________________ Term 2 Practice Final
1.
When a sodium atom becomes a sodium ion it will most likely: a.
gain an electron so that it has more electrons than protons b.
lose an electron so it has more protons than electrons c.
lose a proton so it has fewer protons compared to electrons d.
gain an electron so it has the same number of electrons as protons
2.
How many protons are in an atom of calcium?
3.
How many total electrons are in a neutral calcium atom?
4.
How many valence electrons are in a neutral calcium atom?
5.
Will a calcium atom gain or lose electrons to form a calcium ion?
6.
How many total electrons are in a calcium ion?
7.
How many protons are in a calcium ion?
8.
What is the charge on a calcium ion?
9.
How many main energy levels are occupied by electrons in a neutral calcium atom?
10.
How many main energy levels are occupied by electrons in a calcium ion?
Name these compounds
11. AgNO
12. FeO
13. N
2
O
5
3
Write formulas for these compounds
14. Aluminum nitrate
15. Copper(II)carbonate
16. Phosphorus pentachloride
Balance this equation
17. CuCl
2
+ NaOH Cu(OH)
2
+ NaCl
Use the balanced equation below to answer the following questions:
2 C
2
H
2
+ 5 O
2
4 CO
2
+ 2 H
2
O
18. How many moles of O
2
are needed to completely react with 4.0 moles of C
2
H
2
?
19. If 6.0 moles of C
2
H
2
react with an excess of O
2
, how many moles of CO
2
should be produced?
20. If 2.0 grams of C
2
H
2 react with an excess of O
2
, what mass of H
2
O should be produced?
When iron(III) nitrate reacts with sodium hydroxide, the products are iron(III) hydroxide and sodium nitrate.
21. Write a balanced chemical equation for the word equation above.
Use the balanced equation you just wrote to solve the next problem.
22. If 2.00 grams of iron(III) nitrate react with an excess of sodium hydroxide, what mass of iron(III) hydroxide can be produced?
23. When baking soda is heated it decomposes according to the unbalanced equation shown below. Balance the equation.
NaHCO
3
Na
2
CO
3
+ CO
2
+ H
2
O
(baking soda) (sodium carbonate) (gas) (gas)
a.
A student places an empty crucible on the balance and records the mass. b.
The student then scoops some baking soda into the crucible and records the mass. c.
The student heats the crucible. d.
After cooling, the student places the crucible with the product in it on the balance and records the mass.
24. Fill in the blank cells in the Data Table below
Mass of empty, dry crucible
Mass of crucible with baking soda (before reaction)
Mass of baking soda
Mass of crucible + sodium carbonate
Actual mass of sodium carbonate
14.00 grams
15.00 grams
14.60 grams
25. Based on the amount of baking soda used in the reaction, what theoretical mass of sodium carbonate should have been produced, assuming the reaction went to completion?
26. What could account for the difference between the theoretical mass and actual mass?