Soda Ash Lab Packet - Worcester Think Tank
advertisement

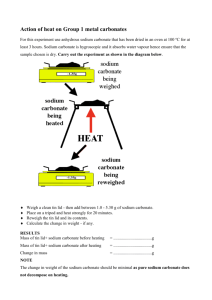
Name ____________________! Data Sheet Item Data A. Mass of NaH2CO3 (going into test tube) _____._____ g B. Mass of sample (after heating in test tube) _____._____ g C. Mass loss _____._____ g D. Mass loss percentage _____% E. Mass of crucible and lid (after heated to reduce impurities) _____._____ g F. Mass of crucible, lid and NaH2CO3 sample (before heating) _____._____ g G. Mass of NaH2CO3 _____._____ g H. Mass of crucible, lid and sample (after heating) _____._____ g I. Mass of sample _____._____ g J. Mass loss _____._____ g K. Mass loss percentage _____% Sample NaH2CO3 (store bought): baking soda NaCO3 (store bought): soda ash Test tube sample (after heating) Crucible sample (after heating) pH Reading (estimated number) Name ____________________! Steps and Procedures Test Tube Heating 1. Measure out ~2.50g of sodium hydrogen carbonate (baking soda). Put this sample into a test tube. 2. Secure a pair of tongs and hold the test tube above a lit alcohol lamp. 3. Hold the test tube and sample in the flame. Try to move the flame evenly along the sample. Heat for 20 to 40 seconds. 4. Before removing the sample from the flame, have a lab partner ready with a glowing splint. 5. Remove the test tube from the flame and have your lab partner hold the glowing splint over the tube. Make an observation. Does it go out? Does it continue to burn? If it continues to burn, hold the test tube over the flame for 20-40 seconds more. 6. As you heat the sample you should notice a liquid condensing on the cooler inside upper surface of the test tube. 7. If necessary try the glowing splint test again. If the flame goes out, it is a good indication that carbon dioxide is outgassing. 8. Put the powder sample into a dish and weigh it. Label your sample for a later step in the lab. 9. Determine the mass loss and mass loss percentage on your Work Problems sheet. This can be done at completion of the lab. Steps and Procedures Crucible Heating 1. Place a covered crucible on a clay triangle and supporting ring. Light an alcohol lamp underneath it and heat it strongly for at least a minute. This will drive off water or other volatile contaminants present. Let it then cool completely. Pleas wait a full 5 minutes. 2. After allowing to cool completely, but still using a protective fabric or plastic dish, weigh the crucible and lid and record the mass. 3. Add approximately ~5.00g of sodium hydrogen carbonate (baking soda) to the crucible and weigh the crucible, lid and contents. Record the mass. 4. Place the covered crucible in the clay triangle. Position the crucible lid in such a way that gases will be allowed to vent. Heat the crucible strongly for 15 minutes. Once the 15 minutes is up, allow the crucible to cool completely. 5. After allowing to cool completely, but still using a protective fabric or plastic dish, weigh the crucible, lid and contents. Record the mass. 6. Determine the weight of the sample. Label your sample for a later step in the lab. 7. Determine the mass loss and mass loss percentage on your Work Problems sheet. This can be done at completion of the lab (after the pH tests). Name ____________________! Steps and Procedure pH Tests One way to determine whether you have successfully decomposed sodium hydrogen carbonate to sodium carbonate is by running a pH test. Please follow the steps and procedures. 1. Get 4 test tubes and label or code them as: a. NaH2CO3 (baking soda) store bought b. NaCO3 (soda ash) store bought c. NaCO3 laboratory made (test tube and heated 30 s) d. NaCO3 laboratory made (crucible and heated 15 min) 3. Fill each test tube with 2 mL of distilled water. 4. Add 1.25g of each sample into the test tube and swirl the solution. 5. Once mostly dissolved (some solute is okay), put a pH strip in the tube. 6. Wait 30 seconds and using the pH chart record the pH reading. You will have to estimate. Name ____________________! Work Problems Calculating Mass Loss Percentage In this experiment you have been asked to calculate the mass loss percentage in the heating of sodium hydrogen carbonate (baking soda). This loss of mass is caused by the outgassing of carbon dioxide and water vapor. Please follow the steps below to complete the mass loss percentage for sodium hydrogen carbonate heated in the test tube and sodium hydrogen carbonate heated in the crucible. Problem 1: NaH2CO3 heated in test tube (____s) 1. Calculate the mass loss by subtracting the sodium carbonate product (line B) from the mass of the sodium hydrogen carbonate reactant (line A). Enter the mass loss on line C if you have not already. 2. Calculate the mass loss percentage by multiplying the mass loss (C) by 100, then dividing this number by the original sodium hydrogen carbonate reactant (A). This is your mass loss percentage. Enter the % in line D. (Show all work) Problem 2: NaH2CO3 heated in crucible (____s) 1. Calculate the mass loss by subtracting the sodium carbonate product (line I) from the mass of the sodium hydrogen carbonate reactant (line G). Enter the mass loss on line J if you have not already. 2. Calculate the mass loss percentage by multiplying the mass loss (J) by 100, then dividing this number by the original sodium hydrogen carbonate reactant (G). This is your mass loss percentage. Enter the % in line K. (Show all work) Name ____________________! Mass Loss Percentage Example: Sodium Hydrogen Carbonate sample (before heating): 12 grams Mass of sample after heating: 8.8 grams Mass loss = 3.2 grams Mass loss percentage calculation 3.2 x 100 = 320 320/10 = 32%