H midyear review ch 4
Logged in as tkoby@whr.nj.
WebAssign.net
Logout
Sunday, January 16, 2005 07:02 PM EST
Home | My Assignments | Grades | Communication | Calendar
Home > My Assignments > H midyear
review ch 4 (Homework)
Guide | Help | My Options
Timothy Koby
ChemistryH, section Period 11, 20042005
Instructor: Victoria Hubinger
Watchung Hills Regional High School
About this Assignment
Due: Monday, January 24, 2005 07:23 AM
EST
Current Score: 143 out of 143
Question Score
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 2/15
4 1/1 1/15
5 1/1 1/15
5/5
Viewing:
Last
Response
View:
All
Responses
Notes
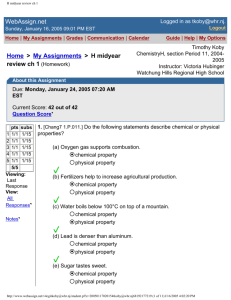
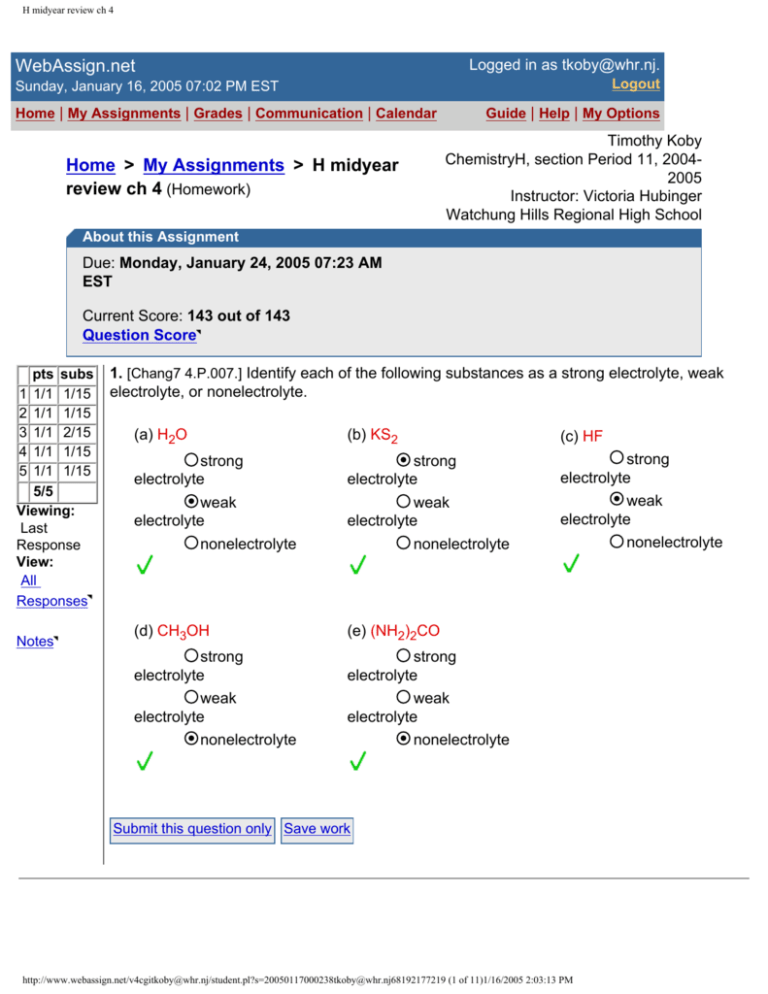
1. [Chang7 4.P.007.] Identify each of the following substances as a strong electrolyte, weak
electrolyte, or nonelectrolyte.
(a) H2O
(b) KS2
(c) HF
strong
electrolyte
weak
electrolyte
nonelectrolyte
strong
electrolyte
weak
electrolyte
nonelectrolyte
strong
electrolyte
weak
electrolyte
nonelectrolyte
(d) CH3OH
(e) (NH2)2CO
strong
electrolyte
weak
electrolyte
nonelectrolyte
strong
electrolyte
weak
electrolyte
nonelectrolyte
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (1 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
pts subs
1 1/1 2/15
2 1/1 2/15
3 1/1 1/15
4 1/1 2/15
4/4
Viewing:
Last
Response
View:
All
Responses
2. [Chang7 4.P.015.] Characterize the following compounds as soluble or insoluble in water.
(b) Ca3PO4
(a) NaOH
soluble
insoluble
(c) Mn(OH)2
soluble
insoluble
(d) AgCl
soluble
insoluble
soluble
insoluble
Submit this question only Save work
Notes
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
28
29
30
pts subs
1/1 1/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 3/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 3/15
1/1 1/15
1/1 6/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 2/15
1/1 1/15
3. [Chang7 4.P.029.] Balance the following equations and write the corresponding ionic and
net ionic equations (if appropriate). (Type your answer using the format [NH4]+ for NH4+.
Use the lowest possible coefficients.)
(a) HBr(aq) + NH3(aq)
ionic equation
1
[H]+
(aq) + 1
(aq) + 1
net ionic equation
[H]+
Br -(aq) + 1
NH3(aq)
1
Br -(aq)
[NH4]+
1
(HBr is a strong acid)
(aq) + 1
NH3(aq)
1
[NH4]+
(aq)
(b) Ba(OH)2(aq) + H3PO4(aq)
ionic equation
3
[Ba]2+
(aq) + 6
OH -(aq) + 2
1
Ba3(PO4)2
(s) + 6
H2O
H3PO4(aq)
(l)
net ionic equation
3
[Ba]2+
(aq) + 6
OH -(aq) + 2
1
Ba3(PO4)2
(s) + 6
H2O
H3PO4(aq)
(l)
(c) HClO4(aq) + Mg(OH)2(s)
ionic equation
2
2
[H]+
OH -(aq)
(aq) + 2
1
[Mg]2+
ClO4-(aq) + 1
(aq) + 2
Mg2+(aq) +
ClO4-(aq) + 2
H2O
(l)
net ionic equation
1
[H]+
(aq) + 1
OH -(aq)
1
H2O
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (2 of 11)1/16/2005 2:03:13 PM
(l)
H midyear review ch 4
31
32
33
34
35
36
37
38
39
40
41
42
43
1/1 2/15
1/1 1/15
1/1 2/15
1/1 1/15
1/1 2/15
1/1 2/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
43/43
Viewing:
Last
Response
View:
All
Responses
Submit this question only Save work
Notes
1
2
3
4
5
6
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
pts subs
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 2/15
1/1 1/15
1/1 6/15
1/1 2/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
4. [Chang7 4.P.039.] For the complete redox reactions given below, do each of the
following. (Type your answer using the format [NH4]+ for NH4+. Also, type [e]- for e-. Use
the lowest possible coefficients.)
(i) Break down each reaction into its half-reactions.
(ii) Identify the oxidizing agent.
(iii) Identify the reducing agent.
(a) 2 Sr + O2
2 SrO
(i) half reactions
1
Sr
1
O2
1
+ 4
Sr2+ + 2
e-
2
[e][O]2-
(ii) oxidizing agent
O2
Sr
(iii) reducing agent
O2
Sr
(b) 2 Li + H2
2 LiH
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (3 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
24
25
26
27
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 2/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 1/15
1/1 2/15
1/1 1/15
1/1 1/15
1/1 3/15
1/1 2/15
1/1 2/15
1/1 1/15
1/1 1/15
48/48
Viewing:
Last
Response
View:
All
Responses
Notes
(i) half reactions
1
Li
1
H2
1
Li+ + 1
e-
+ 2
2
[e][H]-
(ii) oxidizing agent
H2
Li
(iii) reducing agent
H2
Li
(c) 2 Cs + Br2
2 CsBr
(i) half reactions
1
Cs
1
Br2
1
Cs+ + 1
e-
+ 2
2
[e][Br]-
(ii) oxidizing agent
Cs
Br2
(iii) reducing agent
Cs
Br2
(d) 3 Mg + N2
Mg3N2
(i) half reactions
1
Mg
1
N2
1
+ 6
Mg2+ + 2
e-
2
[e][N]3-
(ii) oxidizing agent
Mg
N2
(iii) reducing agent
Mg
N2
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (4 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 3/15
5 1/1 2/15
6 1/1 1/15
7 1/1 1/15
8 1/1 1/15
9 1/1 1/15
10 1/1 1/15
11 1/1 1/15
12 1/1 1/15
13 1/1 1/15
14 1/1 2/15
14/14
Viewing:
Last
Response
View:
All
Responses
5. [Chang7 4.P.043.] Give the oxidation number of the underlined atoms in the following
molecules and ions.
(a) IF7
(b) NaHCO3
7
4
(e) Li2
(f) KMnO4
0
7
(i) C2H4
-2
(j) K2Cr2O7
6
(m) CH4
-4
(c) NaIO3
5
(d) KAuCl4
3
(g) Cs2O
1
(h) PF65
(k) K2CrO4
(l) ClF
1
6
(n) C2H2
-1
Notes
Submit this question only Save work
pts subs
1 1/1 2/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
4/4
Viewing:
Last
Response
View:
All
Responses
6. [Chang7 4.P.051.] Classify the following redox reactions.
(a) 2 H2O2
2 H2O + O2
combination reaction
decomposition reaction
displacement reaction
disproportionation
reaction
(b) Mg + 2 AgNO3
Mg(NO3)2 + 2 Ag
combination reaction
decomposition reaction
displacement reaction
disproportionation reaction
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (5 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
(c) NH4NO2
N2 + 2 H2O
combination reaction
decomposition reaction
displacement reaction
disproportionation
reaction
(d) H2 + Br2
2 HBr
combination reaction
decomposition reaction
displacement reaction
disproportionation reaction
Submit this question only Save work
pts subs
1 1/1 3/15
2 1/1 2/15
3 1/1 1/15
3/3
Viewing:
Last
Response
View:
All
Responses
7. [Chang7 4.P.059.] Calculate the molarity of each of the following solutions.
Notes
Submit this question only Save work
pts subs
1 1/1 2/15
2 1/1 1/15
3 1/1 1/15
4 1/1 2/15
5 1/1 1/15
5/5
Viewing:
Last
Response
View:
All
Responses
8. [Chang7 4.P.062.] Determine how many grams of each of the following solutes would be
needed to make 5.00 102 mL of a 0.500 M solution.
Notes
(a) 29.0 g of ethanol (C2H5OH) in 513 mL of solution
1.23
M
(b) 14.9 g of sucrose (C12H22O11) in 56.0 mL of solution
.778
M
(c) 7.00 g of sodium chloride (NaCl) in 90.7 mL of solution
1.31
M
(a) cesium iodide (CsI)
65.0
g
(b) sulfuric acid (H2SO4)
24.5
g
(c) sodium carbonate (Na2CO3)
26.5
g
(d) potassium dichromate (K2Cr2O7)
73.5
g
(e) potassium permanganate (KMnO4)
39.5
g
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (6 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
Submit this question only Save work
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
5 1/1 1/15
6 1/1 1/15
6/6
Viewing:
Last
Response
View:
All
Responses
9. [Chang7 4.P.075.] How many grams of NaCl are required to precipitate most of the Ag+ ions
from 4.40 102 mL of 0.0113 M AgNO3 solution?
.291
g
Write the net ionic equation for the reaction. (Type your answer using the format [NH4]+ for
NH4+. Use the lowest possible coefficients.)
1
[Ag]+
(aq) + 1
Cl -(aq)
1
AgCl
(s)
Submit this question only Save work
Notes
pts subs
1 1/1 2/15
2 1/1 2/15
3 1/1 1/15
3/3
Viewing:
Last
Response
View:
All
Responses
Notes
10. [Chang7 4.P.079.] Calculate the volume in mL of a 1.420 M NaOH solution required to
titrate the following solutions.
(a) 65.00 mL of a 2.430 M HCl solution
111.2
mL
(b) 25.00 mL of a 3.500 M H2SO4 solution
123.2
mL
(c) 75.00 mL of a 1.500 M H3PO4 solution
237.7
mL
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (7 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
Notes
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
Notes
11. [Chang7 4.TB.102.] Calculate the concentration of the acid (or base) remaining in solution
when 10.7 mL of 0.211 M HNO3 are added to 16.3 mL of 0.258 M NaOH.
0.235 M
0.240 M
0.0174 M
0.0722 M
Submit this question only Save work
12. [Chang7 4.TB.110.] Using the apparatus shown in Figure 4.1, a student found that a
sulfuric acid solution caused the light bulb to glow brightly. However, after the addition of a
certain amount of a barium hydroxide [Ba(OH)2] solution, the light began to dim even though
Ba(OH)2 is also a strong electrolyte. Why?
Adding acids to bases always reduces the conductivity of aqueous solutions.
Adding barium hydroxide to sulfuric acid produces insoluble barium sulfate and
water, which is non-conducting.
Barium sulfate, which is formed in the process described, increases the electrical
resistance of water.
Adding barium hydroxide to sulfuric acid dilutes the solution, which reduces its
electrical conductivity.
Figure 4.1
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (8 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
pts subs
1 1/1 3/15
1/1
Viewing:
Last
Response
View:
All
Responses
Notes
13. [Chang7 4.TB.124.] A useful application of oxalic acid is the removal of rust (Fe2O3) from,
say, bathtub rings according to the following reaction.
Fe2O3(s) + 6 H2C2O4(aq)
2 Fe(C2O4)(aq) + 3 H2O + 6 H+(aq)
Calculate the number of grams of rust that can be removed by 5.00
solution of oxalic acid.
7.98 g
1.33 g
2.88 g
13.3 g
102 mL of a 0.100 M
Submit this question only Save work
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
14. [Chang7 4.TB.040.] What is the reducing agent in the following redox reaction?
4 Fe + 3 O2
2 Fe2O3
O2
O2Fe
Fe3+
Notes
Submit this question only Save work
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
15. [Chang7 4.TB.024.] Which of the following is a characteristic of an acid-base neutralization
reaction?
A base is produced.
A salt is produced.
An acid is produced.
A molecular compound is produced.
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (9 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
16. [Chang7 4.TB.026b.] Which of the following is a weak base?
NaOH
NH3
LiOH
HCOOH (formic acid)
Submit this question only Save work
Notes
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
17. [Chang7 4.TB.026a.] Which of the following is a weak acid?
HCOOH
NH3
HNO3
HBr
Submit this question only Save work
Notes
pts subs
1 1/1 3/15
1/1
Viewing:
Last
Response
View:
All
Responses
18. [Chang7 4.TB.020a.] With reference to Table 4.2, which of the following methods would
separate K+ from Ag+? All cations are assumed to be in aqueous solution, and the common
anion is the nitrate ion.
Add sugar.
Add chloride ions.
Add nitrate ions.
Add iodide ions.
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (10 of 11)1/16/2005 2:03:13 PM
H midyear review ch 4
Table 4.2
Submit this question only Save work
Submit all questions for grading
Save all work
Home My Assignments
WebAssigntm 4.0 © 1997-2005 by North Carolina State University. All rights reserved.
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?s=20050117000238tkoby@whr.nj68192177219 (11 of 11)1/16/2005 2:03:13 PM