H midyear review ch 1
Logged in as tkoby@whr.nj.
WebAssign.net
Logout
Sunday, January 16, 2005 09:01 PM EST
Home | My Assignments | Grades | Communication | Calendar
Home > My Assignments > H midyear
review ch 1 (Homework)
Guide | Help | My Options
Timothy Koby
ChemistryH, section Period 11, 20042005
Instructor: Victoria Hubinger
Watchung Hills Regional High School
About this Assignment
Due: Monday, January 24, 2005 07:20 AM
EST
Current Score: 42 out of 42
Question Score
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
5 1/1 1/15
5/5
Viewing:
Last
Response
View:
All
Responses
Notes
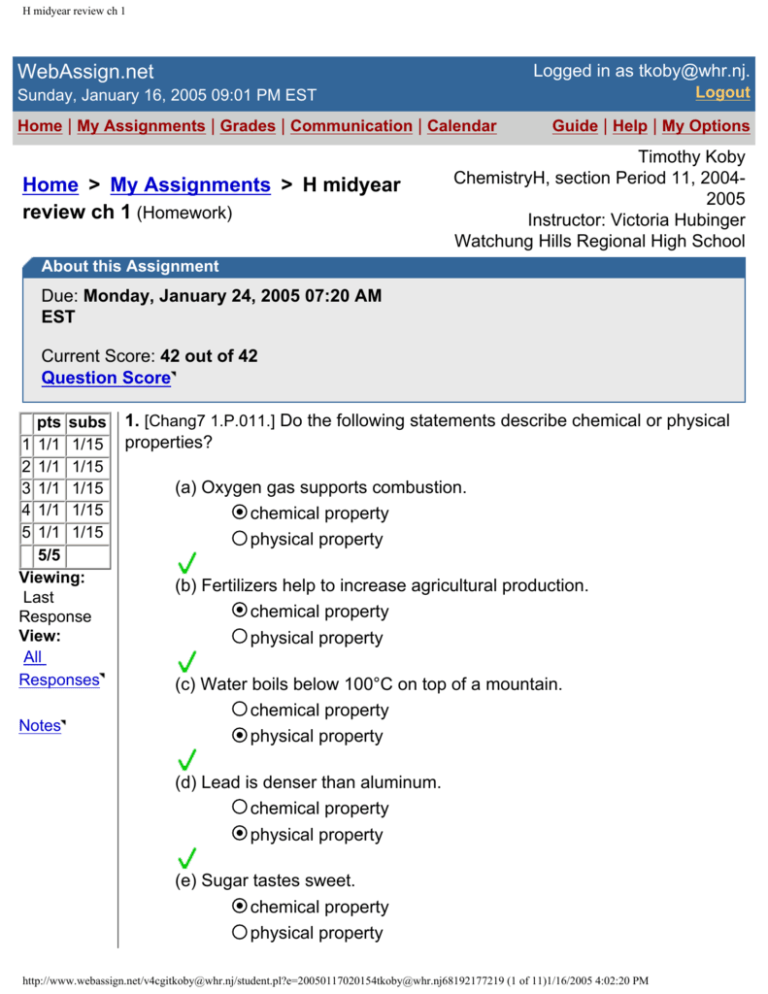
1. [Chang7 1.P.011.] Do the following statements describe chemical or physical
properties?
(a) Oxygen gas supports combustion.
chemical property
physical property
(b) Fertilizers help to increase agricultural production.
chemical property
physical property
(c) Water boils below 100°C on top of a mountain.
chemical property
physical property
(d) Lead is denser than aluminum.
chemical property
physical property
(e) Sugar tastes sweet.
chemical property
physical property
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (1 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
Submit this question only Save work
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
4/4
Viewing:
Last
Response
View:
All
Responses
2. [Chang7 1.P.015.] Classify each of the following substances as an element or
a compound.
(a) dry ice
element
compound
(b) carbon dioxide gas
element
compound
(c) water
Notes
element
compound
(d) hydrogen
element
compound
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (2 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
3. [Chang7 1.P.022.] Mercury is the only metal that is a liquid at room
temperature. Its density is 13.6 g/mL. How many grams of mercury will occupy
a volume of 86.1 mL?
1170
g
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
2 1/1 1/15
3 1/1 1/15
4 1/1 1/15
4/4
Viewing:
Last
Response
View:
All
Responses
4. [Chang7 1.P.023.] Convert the following temperatures to degrees Celsius.
(a) 95°F, the temperature on a hot summer day
35
°C
(b) 17°F, the temperature on a cold winter day
-8.3
°C
(c) a 123°F fever
51
°C
(d) a furnace operating at 1943°F
1062
°C
Notes
Submit this question only Save work
pts subs
1 1/1 1/15
2 1/1 4/15
3 1/1 1/15
3/3
Viewing:
Last
Response
View:
All
Responses
5. [Chang7 1.P.036.] Carry out the following operations as if they were
calculations of experimental results, and express each answer in the correct
units with the correct number of significant figures.
(a) 7.300 km
6.10 km
1.20
(b) (2.66
10-3 mg) - (8.68
.00257
(c) (4.32
106
88700000
mg
dm) + (8.44
10-5 mg)
107 dm)
dm
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (3 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
Notes
Submit this question only Save work
pts subs
1 1/1 3/15
2 1/1 1/15
2/2
Viewing:
Last
Response
View:
All
Responses
6. [Chang7 1.P.069.] The total volume of seawater is 1.5 1021 L. Assume that
seawater contains 3.1% sodium chloride by mass and that its density is 1.03 g/
mL. Calculate the total mass of sodium chloride in kilograms and in tons. (1 ton
= 2000 lb; 1 lb = 453.6 g)
48000000000000000000
kg
53000000000000000
tons
Submit this question only Save work
Notes
pts subs
1 1/1 3/15
2 1/1 3/15
2/2
Viewing:
Last
Response
View:
All
Responses
Notes
7. [Chang7 1.P.075.] Percent error is often expressed as the absolute value of
the difference between the true value and the experimental value, divided by
the true value.
|true value - experimental value|
percent error
100%
=
|true value|
The vertical lines indicate absolute value. Calculate the percent error for the
following measurements.
(a) The density of alcohol (ethanol) is found to be 0.772 g/mL.
(True value is 0.798 g/mL.)
3.26
% error
(b) The mass of gold in an earring is analyzed to be 0.788 g. (True
value is 0.864 g.)
8.80
% error
Submit this question only Save work
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (4 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
8. [Chang7 1.P.079.] Chalcopyrite, the principal ore of copper (Cu), contains
34.63% Cu by mass. How many grams of Cu can be obtained from 5.80 103
kg of the ore?
2010000
g
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
2 1/1 1/15
2/2
Viewing:
Last
Response
View:
All
Responses
9. [Chang7 1.P.087.] Comment on whether each of the following is a
homogeneous mixture or a heterogeneous mixture.
(a) orange juice
homogeneous
heterogeneous
(b) blood
homogeneous
heterogeneous
Notes
Submit this question only Save work
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
10. [Chang7 1.TB.006b.] Water is an example of a compound.
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (5 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
11. [Chang7 1.TB.007a.] The boiling point of a liquid is an example of a physical
property.
true
false
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
12. [Chang7 1.TB.007b.] The density of a substance is an example of a chemical
property.
true
false
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
13. [Chang7 1.TB.008a.] Which of the following is an intensive property?
density
mass
volume
length
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (6 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
14. [Chang7 1.TB.008b.] Which of the following is an extensive property?
boiling point
density
freezing point
volume
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
15. [Chang7 1.TB.012.] Which of the following is a chemical change?
a flashlight beam slowly dims and goes out
helium gas leaking from a balloon
a spoonful of salt is dissolved in a bowl of soup
frozen orange juice is reconstituted by the addition of water
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
16. [Chang7 1.TB.017a.] Which of the following is not an SI base unit?
second
kelvin
kilogram
kilometer
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (7 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
17. [Chang7 1.TB.017b.] Which of the following SI base units is not commonly
used in chemistry?
kelvin
kilogram
candela
mole
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
18. [Chang7 1.TB.018.] Which of the following prefixes means 1/1000?
kilo
deci
milli
centi
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
19. [Chang7 1.TB.021.] If 586 g of bromine occupies 188 mL, what is the density
of bromine in g/mL?
3.12 g/mL
0.312 g/mL
31.2 g/mL
4.53 g/mL
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (8 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
20. [Chang7 1.TB.029a.] What is 0.000000027 expressed in scientific notation?
2.7 108
2.7 10-8
27 10-9
2.7 10-7
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
21. [Chang7 1.TB.029b.] What is 356 expressed in scientific notation?
3.56
3.56
3.56
35.6
102
103
10-2
101
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
22. [Chang7 1.TB.030a.] What is 1.52
10-2 expressed as a decimal?
0.152
0.0152
1520
152
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (9 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
23. [Chang7 1.TB.033.] Which of the following measurements has five significant
figures?
4867 mi
56 mL
0.00003 cm
60,104 ton
Submit this question only Save work
Notes
pts subs
1 1/1 1/15
1/1
Viewing:
Last
Response
View:
All
Responses
24. [Chang7 1.TB.035.] What is 5.6792 m + 0.6 m + 4.33 m expressed in the
correct units with the correct number of significant figures?
10.6
10.60 m
10.6 m
10.6092 m
Submit this question only Save work
Notes
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
25. [Chang7 1.TB.036.] What is 7.310 km 5.70 km expressed in the correct
units with the correct number of significant figures?
1.28 km
1.282 km
1.28
1.282
Submit this question only Save work
Notes
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (10 of 11)1/16/2005 4:02:20 PM
H midyear review ch 1
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
26. [Chang7 1.TB.040.] How many seconds are there in a solar year (365.24
days), expressed in the correct number of significant figures?
3.1 107 s
3.1557 107 s
3.1557 108 s
5.2595 105 s
Submit this question only Save work
Notes
pts subs
1 1/1 2/15
1/1
Viewing:
Last
Response
View:
All
Responses
Notes
27. [Chang7 1.TB.076.] The natural abundances of elements in the human body,
expressed as percent by mass, are: oxygen (O), 65 percent; carbon (C), 18
percent; hydrogen (H), 10 percent; nitrogen (N), 3 percent; calcium (Ca), 1.6
percent; phosphorus (P), 1.2 percent; all other elements, 1.2 percent. What is
the mass (in grams) of oxygen, carbon and hydrogen in the body of a 62 kg
person?
6.5 104 g O; 1.1 104 g C; 8.8 103 g H
4.0 104 g O; 1.1 104 g C; 6.2 103 g H
2.2 104 g O; 3.6 104 g C; 6.2 103 g H
8.0 104 g O; 1.9 104 g C; 5.5 103 g H
Submit this question only Save work
Submit all questions for grading
Save all work
Home My Assignments
WebAssigntm 4.0 © 1997-2005 by North Carolina State University. All rights reserved.
http://www.webassign.net/v4cgitkoby@whr.nj/student.pl?e=20050117020154tkoby@whr.nj68192177219 (11 of 11)1/16/2005 4:02:20 PM