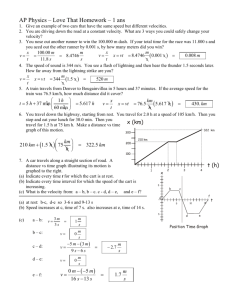
J O2 fO1i 3 \( 2L)
advertisement

______ ____ o1 I— J Name: Hour: Date: Chemistry: Graham’s Law Do the following problems, showing your work and including al/proper units. 10 at the same H 4 C 1. If neon gas travels at 400 m/s at a given temperature, calculate the velocity of butane, , temperature. -- /-/ , has a wintergreen odor, 3 0 H 8 S, has a very strong rotten egg odor. Methyl salicylate, C 2 2. Hydrogen sulfide, H odor. If the vapors for these three substances were pleasant almond 0, has a 6 H 7 and benzaldehyde, C c released at the same time from across a room, which odor would you smell first? Show your work and lain your answer 7/?.Ci) 0 9 i) (1 o/ 7J) 2 3 fO i 1 iT2 / 3. A nitrogen molecule travels at about 505 m/s at room temperature. Find the velocity of a helium atom at the same temperature. JV \J /Lf,oI) - \JLo3 7,3 Sr’ 4. A carbon dioxide molecule travels at 45.0 m/s at velocity of an oxygen molecule. - . 2 O /lCL2 \( 2L) ‘ROc,L,l 5. Nitrogen gas effuses through an opening 1.59 times faster than does an unknown gas. a. Calculate the molecular mass of the unknown gas. 2 N >Qz 7o,t’ iJ b / (0 C/i)o \J5oz b. Make a reasonable prediction as to what the unknown gas is. 6. An unknown gas diffuses 1.62 times slower than does oxygen gas. a. Calculate the molecular mass of the unknown gas. 2 O / 1-z b. Make a reasonable prediction as to what the unknown gas is. Answers: 1 2. 3. 236 rn/s S 2 H 1336 mIs 4. 5a. 5b. 52.8 mIs 71 a.m.u. 2 Cl 6a. 6b. 84 a.m.u. Kr