Graham's Law
advertisement

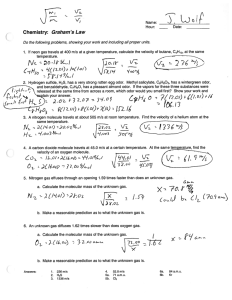
Name: ________________________ Hour: ____ Date: ___________ Chemistry: Graham’s Law Do the following problems, showing your work and including all proper units. 1. If neon gas travels at 400 m/s at a given temperature, calculate the velocity of butane, C4H10, at the same temperature. 2. Hydrogen sulfide, H2S, has a very strong rotten egg odor. Methyl salicylate, C8H8O3, has a wintergreen odor, and benzaldehyde, C7H6O, has a pleasant almond odor. If the vapors for these three substances were released at the same time from across a room, which odor would you smell first? Show your work and explain your answer. 3. A nitrogen molecule travels at about 505 m/s at room temperature. Find the velocity of a helium atom at the same temperature. 4. A carbon dioxide molecule travels at 45.0 m/s at a certain temperature. At the same temperature, find the velocity of an oxygen molecule. 5. Nitrogen gas effuses through an opening 1.59 times faster than does an unknown gas. a. Calculate the molecular mass of the unknown gas. b. Make a reasonable prediction as to what the unknown gas is. 6. An unknown gas diffuses 1.62 times slower than does oxygen gas. a. Calculate the molecular mass of the unknown gas. b. Make a reasonable prediction as to what the unknown gas is. Answers: 1. 2. 3. 236 m/s H2S 1336 m/s 4. 5a. 5b. 52.8 m/s 71 a.m.u. Cl2 6a. 6b. 84 a.m.u. Kr KEY Chemistry: Graham’s Law Do the following problems, showing your work and including all proper units. 1. If neon gas travels at 400 m/s at a given temperature, calculate the velocity of butane, C 4H10, at the same temperature. Neon m2 20.2 g v2 400 m/s Unknown m1 58 g v1 ? m/s v1 v2 m2 m1 v1 400 m/s 20.2 g 58 g v1 236 m/s 2. Hydrogen sulfide, H2S, has a very strong rotten egg odor. Methyl salicylate, C8H8O3, has a wintergreen odor, and benzaldehyde, C7H6O, has a pleasant almond odor. If the vapors for these three substances were released at the same time from across a room, which odor would you smell first? Show your work and explain your answer. H2 S 34 amu C 8 H8 O 3 C 7H6 O 152 amu 106 amu All substances have the same kinetic energy (all are at the same temperature). Therefore, the lightest molecules will move fastest. 3. A nitrogen molecule travels at about 505 m/s at room temperature. Find the velocity of a helium atom at the same temperature. N2 m2 28 g v2 505 m/s Helium m1 4g v1 ? m/s v1 v2 m2 m1 v1 505 m/s 28 g 4g v1 1336 m/s 4. A carbon dioxide molecule travels at 45.0 m/s at a certain temperature. At the same temperature, find the velocity of an oxygen molecule. CO 2 O2 m2 44 g v2 45 m/s m1 32 g v1 ? m/s v1 v2 m2 m1 v1 45 m/s 44 g 32 g v1 52.8 m/s 5. Nitrogen gas effuses through an opening 1.59 times faster than does an unknown gas. a. Calculate the molecular mass of the unknown gas. Nitrogen Unknown m2 v2 28 amu 1.59 x m1 58 amu v1 1x v1 v2 m2 m1 1.59 x 1x m2 28 g b. Make a reasonable prediction as to what the unknown gas is. Chlorine gas, Cl2 2 Cl @ 35.453 amu = 70.9 amu m2 70.8 amu KEY – Page 2 Chemistry: Graham’s Law 6. An unknown gas diffuses 1.62 times slower than does oxygen gas. a. Calculate the molecular mass of the unknown gas. Oxygen m1 32 amu v2 1.62 x Unknown m2 ? amu v2 1x v1 v2 m2 m1 1.62 x 1x m2 32 amu m2 b. Make a reasonable prediction as to what the unknown gas is. Krypton, Kr Answers: 1. 2. 3. 236 m/s H2S 1336 m/s molecular mass is 83.80 amu 4. 5a. 5b. 52.8 m/s 71 a.m.u. Cl2 6a. 6b. 84 a.m.u. Kr 84 amu