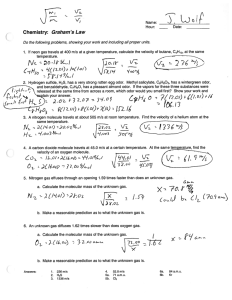
Unit 3 Study Guide Answer Key
advertisement
Name______________________________________ Date______ Block____ Unit 3: Chemistry Learning Target I can identify chemical and physical changes and properties. I can use data to determine if a chemical reaction occurred and describe the change in properties. I can label and identify parts of a chemical equation. I can construct and use a model to explain the Law of Conservation of Mass. Activity Self Check: Have it…or still working on it? 1. Examples of Chemical and Physical Properties Chemical Property Physical Property Flammability Density Odor Melting point Reactivity Boiling point 5. Circle the subscript and put a box around the coefficients 6. Label each part of the equation 2. Examples of Chemical and Physical Changes Chemical Change Physical Change Burning Boiling Bubbling Freezing Odor produced Dissolving New substance formed Breaking 3. Define Chemical and Physical Change Chemical Change: Happens when one or more substances are changed into new substances that have new and different properties. Physical Change: any change NOT involving a change in the substance's chemical identity Produces or yields Reactants Products 7. Balance the chemical equations 4. Using the chart below, determine which test caused a chemical reaction. Explain how you know a chemical reaction took place. Test 1 2 3 4 Substance A White powder Red powder Substance B Clear liquid Clear liquid White powder Yellow liquid Clear liquid Clear liquid Observation Bubbling Color change, no odor produced Odor produced Precipitate formed In Test 1, a chemical reaction took place because bubbling was observed. Bubbling is a sign of a chemical change. In Test 3, a chemical reaction took place because an odor was produced. Production of an odor is a sign of a chemical change. In Test 4, a chemical reaction took place because a precipitate was formed. A new substance formed is a sign of a chemical change. 8. What is the Law of Conservation of Mass? The law of conservation of mass is a law of science that states that MATTER cannot be CREATED or DESTROYED only CHANGED in form. 9. How does the Law of Conservation of Mass apply to chemical reactions? The mass of the reactants must be equal to the mass of the products.