iodometric analysis of vitamin c
advertisement

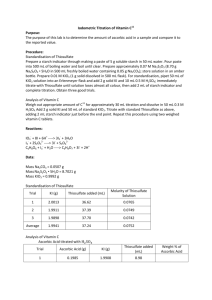
Chemistry 163 Harold IODOMETRIC ANALYSIS OF VITAMIN C Chemistry 163 Laboratory South Puget Sound Community College LEARNING OBJECTIVES The objectives of this experiment are to... 1. prepare a solution of potassium iodate with a precisely known concentration, using primary standard KIO 3 (s). 2. prepare a solution of sodium thiosulfate and standardize it by titration with portions of the above potassium iodate solution. 3. use the iodate and thiosulfate solutions to analyze a vitamin C tablet to determine how many mg of ascorbic acid it contains. BACKGROUND Many analytical methods are based on the redox properties of the iodide ion. In iodometric titrations, excess iodide ion is added to the substance to be analyzed. The iodide ion acts as a reducing agent and is itself oxidized to iodine. The iodine so produced is then titrated with standard sodium thiosulfate solution (Na 2 S 2 O 3 ). In this experiment, potassium iodate (KIO 3 ) is used to standardize the Na 2 S 2 O 3 . When potassium iodide is added to potassium iodate, the following net ionic reaction occurs: IO 3 − + 5 I− + 6 H+ → 3 I2 + 3 H2O Potassium iodide is added in excess so the iodate ion, IO 3 −, is the limiting reactant and, therefore, determines the amount of I2 produced. The I2 formed reacts with excess iodide ion to form the tri-iodide ion, I3 −, which is then titrated with sodium thiosulfate, Na 2 S 2 O 3 . The net ionic equations for these reactions are as follows: I2 + I− → 2 S 2 O 3 2− + I3 − I3 − → S 4 O 6 2− + 3 I− There are three parts to this experiment. The first is preparation of an approximately 0.07 M solution of Na 2 S 2 O 3 and preparation of a 0.02 M solution of KIO 3 whose molarity is accurately known (0.02xxx M). The second part is the standardization of the Na 2 S 2 O 3 solution using the known KIO 3 solution. The final part involves the use of both solutions to analyze a vitamin C tablet to determine its ascorbic acid content. The vitamin C analysis is based on the fact that I3 − oxidizes ascorbic acid as follows: ascorbic acid 04/01/10 Chemistry 163 Harold A known amount of iodate, IO 3 −, is added to excess iodide ion, I−, producing a known amount of I3 −, which then reacts with the ascorbic acid in the vitamin C tablet. The amount of I3 − that does not react with the ascorbic acid is then titrated with the standard Na 2 S 2 O 3 . From the data obtained, the amount of ascorbic acid originally present can be determined. mole I 3 − (from IO 3 -) = mole I 3 − (reacted with vitamin C) + mole I 3 − (titrated with Na 2 S 2 O 3 ) The first quantity above is calculated from the volume and molarity of the KIO 3 solution used; the last quantity is calculated from the volume and molarity of the Na 2 S 2 O 3 solution used in the titration. From these quantities, the moles of I3 − which reacted with the vitamin C can be determined. With this result, the amount of ascorbic acid originally present in the vitamin C tablet can be calculated. EXPERIMENTAL Preparation of Solutions – Day 0 (during Ka Lab period) 1. Preparation of the sodium thiosulfate solution. Place about 550 mL of distilled water in a clean, rinsed 1000 mL beaker, cover with a large watch glass, and boil the water at high heat for at least 5 minutes to destroy bacteria that can cause decomposition of sodium thiosulfate. A stirring rod placed in the beaker helps give an even boiling action and prevents bumping (explosive boiling). Cool briefly, then add about 8.6 g of Na 2 S 2 O 3 and 0.05 g of Na 2 CO 3 , both weighed on a top loader balance. Stir until dissolved, then transfer into a clean plastic bottle. Cap tightly and mix well. 2. While water is heating, weigh about 2.2 g of primary standard KIO 3 into a clean, dry glass weighing bottle, using the top loader balance. Place the weighing bottle (uncovered w/o lid) in a small beaker, write your initials on the beaker, cover the beaker with a watchglass, and dry the KIO 3 in an oven at 110°C for 45 minutes. Afterward, let the beaker and contents initially cool on the lab bench for 5 minutes. Finally, place the weighing bottle and lid in your desiccator for storing until Day 1 of this lab. Standardization of sodium thiosulfate solution – Day 1 and 2 Prepare a KIO 3 solution of known molarity by accurately weighing the dried KIO 3 into a 500.0 mL volumetric flask. Use an analytical balance to determine mass ± 0.0001 grams. First place the weighing bottle with KIO 3 on the balance pan and tare the balance to read zero. Then transfer the KIO 3 to the flask such that 2.1 grams ±0.0001g is transferred. Place the weighing bottle again on the balance pan. The display will give the exact amount transferred. (Weight by difference) The 500.0 mL volumetric flask should first be cleaned and rinsed well with DI water. It does not need to be dry. Use a glass funnel for the transfer process. Tap the funnel to cause downward movement of the KIO 3 and rinse it well. Add about 300 mL of DI water and dissolve the KIO 3 completely. Then carefully dilute to the mark and mix the solution thoroughly by repeated inversion of the capped flask. Calculate the molarity to four significant figures. It should be near 0.02 M ±0.00001. Transfer this solution to a clean plastic bottle and return the cleaned volumetric flask. The transfer process is done as follows: (1) rinse the plastic bottle well with DI water, (2) rinse this bottle several times with small portions (a few mL) of the KIO 3 solution, and (3) transfer all of the remaining KIO 3 solution into the bottle. This procedure eliminates having to dry the bottle and prevents any 04/01/10 Chemistry 163 Harold dilution from occurring. For the following work, you will need your calibrated 25 mL pipet and 50 mL buret. Both need to be cleaned and rinsed so that water drains to leave a uniform film (no drops). See your instructor if you encounter difficulty with cleaning. The pipet needs to be rinsed with several small portions of solution before making a transfer. Similarly, the buret needs to be rinsed with several small solution portions before filling. All buret readings should be estimated to the nearest 0.01 mL and corrected according to your buret correction graph. 1. Pipet 25.00 mL (actual volume is the calibrated value!) samples of standard KIO 3 solution into a 250 mL Erlenmeyer flask. This flask need not be dry prior to the pipeting. Add 25 mL of DI water to each, using a graduated cylinder. From this point, treatment of this first sample must be completed (as described in steps 2 - 4) before the making and treatment of a second flask/sample. Also complete the second before starting the third. 2. To the first sample add approximately 2 g KI (using the top loading balance). 3. Add approximately one mL of 6 M H 2 SO 4 (using a plastic dropper with a one-mL calibration line). The formation of tri-iodide ion should occur, giving the solution a dark red-brown color. 4. Immediately titrate this solution with your thiosulfate solution until the solution color changes gradually from red-brown to medium yellow. Add approximately 3 mL of starch indicator solution (using three additions with a plastic dropper filled to the one-mL calibration line each time) and continue titrating. (If it doesn’t turn blue when starch is added you have gone past the end-point and the trial is invalidated.) Starch and I 3 − form an intensely colored blue compound, and the end-point is reached when the solution color then changes from blue to colorless (this occurs very quickly at end-point). Starch is partially decomposed by large excesses of iodine, so the starch is added only after most of the I3 − has been reduced. Continue titrating until the solution goes colorless and record this end-point volume. After the first titration is complete, you will know about when to add the starch for subsequent titrations. Add the starch as close to the end-point (but not beyond!) as your tolerance for risk allows. 5. Repeat steps 2 through 4 with the second and third samples. The final three titration end-point volumes should closely agree. A fourth and/or fifth titration can be done if the RSD is too high. 6. Calculate the molarity of the sodium thiosulfate solution to four significant figures for each of the determinations. In addition, report the mean, standard deviation, and relative standard deviation for the sodium thiosulfate molarity. Analysis of a Vitamin C Tablet – Day 2 1. Weigh a 100mg vitamin C pill on a sheet of weighing paper on the analytical balance. After weighing carefully grind the pill to a fine powder with a mortar and pestle. Transfer the powder to the weighing paper and re-weigh it. Record this powder weight also. Transfer the powder into a relatively dry 250 mL Erlenmeyer flask. Once the tablet is wet, slow air oxidation will occur. Therefore, complete the analysis as quickly as possible. Thus, do the pills one at a time. Do not get the powder wet until ready to titrate. 2. Add approximately 50 mL of DI water and 2 mL of 6 M H 2 SO 4 , and mix thoroughly. Vitamin C tablets contain an insoluble binder material so the resulting solution will be turbid (look this word 04/01/10 Chemistry 163 Harold up!). 3. Add approximately 2 g of KI to the mixture. 4. Pipet 25.00 mL of standard iodate solution into the flask. Swirl the flask to ensure complete mixing of the contents. 5. Immediately titrate this mixture with your standardized sodium thiosulfate solution as described in step 4 of the standardization procedure. Note that less sodium thiosulfate will be used because of the partial consumption of tri-iodide ion by the ascorbic acid. Some brands of vitamin C tablets use starch as the binder and this starch will also act as an indicator, giving the solution an initial blue color. If this occurs, add the indicator starch anyway (but less is OK). Make this addition when the original blue color is somewhat faded (the exact point is not critical). Also, the binder starch particles tend to irreversibly retain a small amount of tri-iodide ion and will appear dark at the end-point. This effect can be ignored, but is one of the reasons we can only expect 3 S.F. accuracy in the weight of the Vitamin C determination. 6. Calculate the milligrams of ascorbic acid found in your tablet. Your result should be considered valid to 3 significant figures. Complete the analysis in triplicate (3x) for in this experiment. If any problems were encountered, such a overshooting an end-point, you should do another sample. The pill is such that the results should yield ~100 mg of ascorbic acid as indicated by the manufacturer. 7. The reason for weighing the pill before and after grinding is that you likely will not be able to recover 100% of the powder. By dividing powder weight by pill weight you will have an accurate measure of the fraction of the pill you will actually titrate. This fraction divided into the actual mass of Vitamin C determined by titration will “standardize” the Vitamin C mass to what it would have been if 100% of the pill had been used. Thus, we can determine the validity of the manufacturers claim that each pill contains 100 mg of Vitamin C. Recommended strategy The lab day prior to the beginning of this lab (Day 0) you will (1) boil the deionized water and prepare your solution of sodium thiosulfate; (2) dry the sample of pure KIO 3 and prepare its standard solution. Two periods have been allotted for the remainder of this experiment. It is recommended that during the first period you do the following: begin the standardization titrations, using these prepared solutions. The end-point in this titration is tricky, so some experience with it prior to collecting real data is helpful (that is, do at least one practice titration). You likely will not complete the three standardization titrations in the first period. In the second day then will be the completion of the three standardization titrations (if not accomplished) and the three vitamin C pill analyses titrations should also be done as described in the procedure. 04/01/10