Biochemistry –

Biochemistry
THEME – STRUCTURE DICTATES FUNCION
Main Ideas - 1)
2)
3)
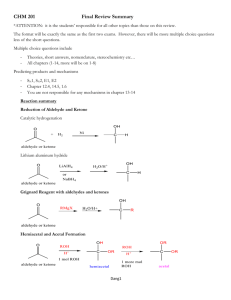
Ch. 10 – Carbohydrates
10.1 Introduction to Carbohydrates –
Carboyhydrates –
Structure
Mono-
- either polyhhydroxyaldehyde or a polyhydroxyketone di- oligo- poly- saccharides
Roles - metabolism of carbohydrates provides energy
- serve as structural and protective materials
- precursor molecules for biosynthesis of proteins, lipids, and nucleic acids
- attached to proteins and lipids in cell membranes to help in cell recognition and specific molecules, triggering physiological processes
- ribose and deoxyribose are the sugars in DNA and RNA
10.2 Monosaccharides
Classification -
1) ketone/aldehyde containing
2) carbon chain length
3 C’s
4 C’s
5 C’s
6 C’s
Build it!
KEY: Be sure to understand that when you “thought” you built glucose it’s highly likely that most people built a stereoisomer of glucose.
1
Stereoisomerism - aldoses & ketoses <<Figures 10.1 and 10.2>>
Isomerism Summary from Ch. 9
Isomers
(Connectivity Differences) (Connectivity Differences)
2
Recognizing structural relations:
For each of the following pairs of compounds, indicate whether the pair consists of different compounds that are (1) constitutional isomers or (2) stereoisomers that are enantiomers or (3) stereoisomers that are diasteromers or (4) not isomers.
(a) D-Glucose and D-mannose
(b) D-ribose and D-xylulose
(c) D-fructose and D-arabinose
(d) D-sorbose and L-sorbose
(e) D-sorbose and D-fructose
Important Monosaccharides
Sugar aldopentose
Practice
Cyclic
Shape
#
Stereoisomers glucose aldotetrose fructose ketotetrose galactose ketohexose
2-deoxy-D-ribose
D-ribose
10.3 Cyclic Hemiacetal Structures
To convert acyclic structure to cyclic structure.
• Draw the fischer projection of the acyclic structure and then turn it sideways.
• Rotate the bond between C4 and C5 to bring the C5-OH group close to the carbonyl group.
• Add the alcohol – OH to the carbonyl group: break the O-H bond, and then bond the H of the –OH to the carbonyl O and bond the O of the –OH to the carbonyl C.
• aldoses exist predominantly as _______________.
• aldhexoses take the shape of a _______________.
3
• Ketoses exist predominantly as ___________________.
• Ketopentoses take the shape of a ___________________.
Practice Drawing Fischer and Haworth projections:
Draw the cyclic structure of a-D-galactose, referring only to figure 10.1. Indicate how this task is simplified if you are allowed to directly refer to both figures 10.1 and 10.3
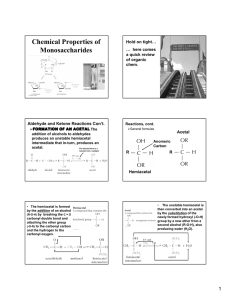
10.4 Chemical and Physical Properties of Monosaccharides
Monosaccharides - solubility in water ________________ phase at room temp. ________________ highly concentration solutions are ____________ solubility in alcohols ________________ solubility in ethers & hydrocarbons ________________ taste ________________
4
Box 10.1 How Sweet Is It?
• Taste depends on _____________, ______________, and _______________. saccharin cyclamates aspartame acesulfame K sucralose
Artificial sweeteners are “calorie-free” because___________________________________________
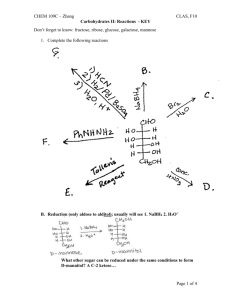
Oxidation of the Aldehyde Group/ Benedicts Test
Aldose + Cu 2+ carboxylic acid + Cu
2
O
• ____________________________ are considered “reducing sugars.”
• Reducing sugars bring about the ____________ of another substance while they get _____________.
• In order to test positively the sugar must be in the _____________ form.
•
-hydroxy ketones like fructose are converted to aldoses in the alkaline Benedicts soln, thus give a
__________ test.
Reducing Sugars NOT Reducing sugars
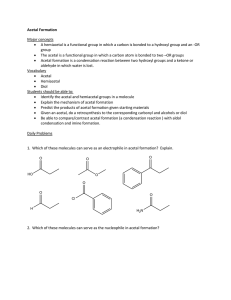
Acetal Formation: The Production of Glycosides
Hemiacetal (aldose/ketose) + alcohol
acetal
Acetal -
NOTE: the
5
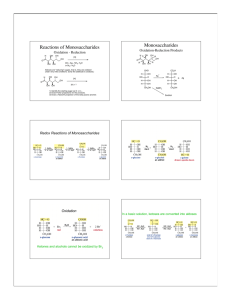
10.5 Disaccharides
Maltose
Cellobiose
Lactose
Sucrose
Understanding the notation:
• the
in
(1
4)- indicates
• the first number in
(1
4)- indicates
• the second number in
(1 4)- indicates
6
Ex. 10.5
–Drawing the structure of a disaccharide
Melibiose, a disaccharide found in some plants, contains D-galactose linked to D-glucose by an
(1 6)-glycosidic linkage – an a-glycosidic linkage from C1 of D-galactose to C6 of D-glucose.
Draw the structure of melibiose and indicate whether it is a reducing sugar. Does it undergo mutarotation?
Digestion and Absorption of Carbohydrates
• Only ________________ are small enough to pass through the intestinal wall into the blood stream. sucrose lactose maltose
10.6 Polysaccharides – starch, glycogen, cellulose
Polysaccharides differ structurally several ways:
1. In the monosaccharide(s) that constitute(s) the residues.
2. In the –OH group(s) that participate(s) in linking the monosaccharide residues.
3. In the glycosidic linkage (
or
), when one of the –OH groups is the hemiacetal –OH (as is usual).
4. In the presence or absence of branching in the polysaccharides.
Starch –
10-30%
30-90%
Glycogen structurally similar to _______________
Digestion:
Storage:
When needed:
Starch glucose + glucose glycogen
7
Cellulose:
• gives the plant ______________ and ______________
• structure:
Stable conformation:
Digestion:
Humans
Iodine Test:
Box 10.4 Dietary Fiber
Dietary Fiber –
•
•
Soluble
• vs. Insoluble
Cell Recognition: Glycolipids and Glycoproteins
Grazing Animals
Cell Recognition – cells recognize one another due to ____________________ attached to the cell surface (usually ________ saccharides).
- saccharides are present as 1)
Ex. Egg – sperm
Blood types
Other Ex’s
10.7 Photosynthesis
2)
Plants can convert carbohydrate to
8