Chapter 6 - Thermochemistry AP Chemistry Thermodynamics
advertisement

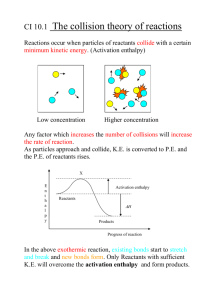
Chapter 6 - Thermochemistry AP Chemistry Thermodynamics • Study of energy and the changes it undergoes • 1st law of thermodynamics – the energy of the universe is constant The Nature of Energy • Energy – capacity to do work or release heat • Law of Conservation of Energy – energy can be converted from one form to another, but can’t be created or destroyed • The energy of the universe is constant Kinetic vs. Potential Energy • Kinetic Energy - energy an object has due to its motion and depends upon the object’s mass and velocity (KE=(1/2)mv2) • Potential Energy – energy due to position or composition Chemical Energy • Chemical Energy – potential energy stored within the bonds of a substance • System – part of the universe we are focusing on (reactant and products in a reaction) • Surroundings – everything else in the universe (room reaction is taking place in) Heat vs. Temperature • Heat – energy transferred between two objects due to temperature differences between them • Temperature – measure of the average kinetic energy of particles in matter Exothermic vs. Endothermic • Exothermic – heat is evolved, or given off; energy flows out of the system • Endothermic – heat is absorbed; energy flows into the system • The energy gained by the surrounding must be equal to the energy lost by the system Exothermic Reactions • Potential energy stored in the chemical bonds is being converted to thermal energy via heat Endothermic Reactions • Thermal energy is being converted into potential energy stored in the chemical bonds Enthalpy • ΔH = E + PV • At constant pressure, ΔH = q, or the change in enthalpy of a system is equal to the energy flow as heat • When studying reactions at constant pressure, the terms heat of reaction and enthalpy are interchangeable • ΔH = ΔHproducts - ΔHreactants Enthalpy Example • How much heat energy is transferred through the combustion of 0.4423 g of butane at constant pressure? C4H10 + O2 CO2 + H2O + 5779 kJ Calorimetry • Science of measuring heat • Calorimeter – device used to measure heat change • Heat Capacity (C) – C = (heat absorbed/increase in temperature) • Specific Heat Capacity (c or s) – amount of heat necessary to raise 1 gram of a substance by 1 oC • Molar Heat Capacity - amount of heat necessary to raise 1 mole of a substance by 1 oC Constant-Pressure Calorimetry • Constant-Pressure – heat of reaction is equal to the enthalpy change • ΔH = q = specific heat x mass x Δtemperature, q = mcΔT Constant-Pressure Calorimetry Example • A 50.0 mL of 0.500 M Ba(NO3)2 solution at 22.0 oC is mixed with 25.0 mL of 1.00 M Na2SO4 in a calorimeter to produce a white precipitate. The temperature of the solution increased to 28.1 oC. Assume this reaction took place at constant pressure and the calorimeter absorbed a negligible amount of heat (Density of the final solution is equal to 1.00 g/mL and its specific heat capacity is 4.18 J/(goC)). Calculate the enthalpy change per mole of BaSO4 formed. Hess’s Law • Enthalpy is a state function – In going from a particular set of reactants to a particular set of products, the change in enthalpy is the same whether the reaction takes place in one step or a series of steps. • Hess’s law is useful to determine the heat of reaction for reactions that are difficult to directly measure in the laboratory. Characteristics of Enthalpy Changes 1. If a reaction is reversed, the sign of ΔH is also reversed. 2. The magnitude of ΔH is directly proportional to the quantities of reactants and products in a reaction. If the coefficients in a balanced reaction are multiplied by a number, the value of ΔH is multiplied by the same number. Example • C(diamond) C(graphite) What is the ∆H associated with this reaction? 1. C(s, graphite) + O2(g) CO2(g) ∆H = -393.5 kJ 2. C(s, diamond) + O2(g) CO2(g) ∆H = -395.4 kJ 1. can be reversed to yield: CO2(g) C(s, graphite) + O2(g) ∆H = 393.5 kJ Add equation 2 and the reverse of equation 1 together to yield: C(s, diamond) + O2(g) CO2(g) ∆H = -395.4 kJ + CO2(g) C(s, graphite) + O2(g) ∆H = 393.5 kJ __________________________________________ C(s, diamond) C(s, graphite) ∆H = -1.9 kJ Hints 1. Work backwards from required reaction. 2. Reverse reactions as necessary to obtain the proper reactants and products. 3. Multiply reactions by numbers to obtain the correct number of reactants and products. Now you try one • Given the following data: C6H4(OH)2(aq) C6H4O2(aq) + H2(g) H2(g) + O2(g) H2O2(aq) H2(g) + 1/2O2(g) H2O(g) H2O(g) H2O(l) • ∆H= +177.4 kJ ∆H= -191.2 kJ ∆H= -241.8 kJ ∆H= -43.8 kJ Calculate ∆H for the reaction: C6H4(OH)2(aq) + H2O2(aq) C6H4O2(aq) + 2H2O(l) Standard Enthalpies of Formation • Change in enthalpy that accompanies the formation of one mole of a compound from its elements with all substances in their standard states. • Degree symbol indicates the process is occurring under standard conditions (ΔHfo) Standard Conditions • For a Compound: – The standard state of a gaseous substance is a pressure of exactly 1 atmosphere. – For a pure substance in a condensed state (liquid or solid), the standard state is the pure liquid or solid. – For a substance present in a solution, the standard state is a concentration of exactly 1M. • For an Element: – The standard state of an element is the form in which the element exists under conditions of 1 atmosphere and 25oC. (The standard state for oxygen is O2(g) at a pressure of 1 atmosphere and 25oC; the standard state for sodium is Na(s); the standard state for mercury is Hg(l)) Heat of Formation Example • Write an equation for the heat of formation for 1 mol of NaCl. Calculating Enthalpy of Formation • The enthalpy of a given reaction can be calculated by subtracting the enthalpies of formation of the reactants from the enthalpies of formation of the products. • ΔHoreaction= ∑np ΔHof(products) - ∑np ΔHof(reactants) Enthalpy of Formation • Determine the heat of the following reaction using the given standard heats of formation. C2H5OH(l) + O2(g) 2 CO2(g) + 3 H2O(g) Compound ∆Hfo(kJ/mol) C2H5OH(l) CO2(g) H2O(g) -278 -394 -242 When Doing Enthalpy Calculations • When a reaction is reversed, the magnitude of ΔH remains the same, but its sign changes. • When the balanced equation for a reaction is multiplied by a factor, the value of ΔH for that reaction must be multiplied by the same factor. • Elements in their standard states are not included in the ΔHof calculations. (ΔHof for an element in its standard state is zero)