Thermo Review
advertisement

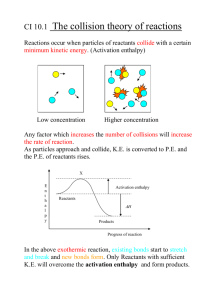
Thermo Review 0. True or false: Energy transfers occur only during chemical reactions False 1. For an endothermic reaction, the overall enthalpy change is a. Negative b. Positive c. Does not have a sign only a unit 2. To write the alternate form of the following reaction H2 + Cl2 2HCl H = - 185 kJ a. Change the sign b. Reverse the equation c. Write the energy as a reactant d. Write the energy as a product H2 + Cl2 2HCl H = - 185 kJ 3. What mass of Chlorine would be required to release 925 kJ of energy? a. cannot be determined b. 5 grams c. 177.25 grams d. 354.5 grams 4. The standard enthalpy change for a reaction can be found by which of the following a. sum of enthalpies of reactants minus sum of enthalpy of products, excluding coefficients b. sum of enthalpies of reactants minus sum of enthalpy of products, including coefficients c. sum of enthalpies of products minus sum of enthalpy of reactants, excluding coefficients d. sum of enthalpies of products minus sum of enthalpy of reactants, including coefficients 5. Specific heat capacity is… a. the energy required to form a compound b. the energy needed to raise a substance one degree celsius c. the energy needed to raise the temperature of one gram of substance one degree celsius d. Always greater than 1 6. How much heat is given off by a 50.0 g sample of copper when it cools from 80.0 to 50.0C? Specific heat of copper is 0.382 J/gC a. - 573 J b. 573 J c. 573 kJ d. - 573 kJ O2(g) + 2SO2 (g) 2SO3 (g) 7. Determine the change in enthalpy of the reaction using the standard heats of formation a. 296.8 kJ b. -395.7 kJ c. -791.4 kJ d.- 197.8 kJ 8. Iron has a specific heat of 0.446 J/gC. When a 7.55 g piece of iron absorbs 10.33 J of heat. If it was originally at room temp. (22.0C), what is the final temperature? a. 3.07 C b. 18.93 C c. 25.1 C 9. Along which portions of the heating/cooling curve is there no temperature change? a) b) c) d) Solid and Liquid phase Solid, Liquid and Gas phase Vaporization and Fusion Solid and Gas phase 10. What happens to the temperature of the water in the calorimeter when a heated metal is put inside? a) b) c) d) Water temperature goes up Water temperature goes down Water temperature stays the same Water temperature goes down and then up 11. If heat is absorbed by a chemical system, then the surroundings have to a) Release heat b) Absorb heat c) Stay at the same temperature 12. Based on what law are we able to determine the specific heat of an unknown metal? a) b) c) d) Law of Conservation of Matter Law of Conservation of Energy Combined Gas Law Avogadro’s Law 13. If the water in a calorimeter absorbs 526J of heat, how much heat did the object that was placed inside release? a) b) c) d) 263J 526J -263J -526J 14. Endothermic reactions: a) b) c) d) Do not involve heat Absorb heat Release heat Happen instantaneously 15. Calculate the amount of heat needed to raise 42.3g sample of water from -35.7ºC to 112.0ºC a) b) c) d) 3160J 26,140J 132, 000J 95,600J